FAQ for the FDA’s New Guidance on Digital Health Technologies
89% of sponsors now embrace decentralized clinical trials (DCTs). With DCTs becoming commonplace, sites, sponsors and CROs have turned to the FDA for guidance on how to use clinical trial technology compliantly.
In December 2021, the FDA responded to this need with the document, “Digital Health Technologies for Remote Data Acquisition in Clinical Investigations: Guidance for Industry, Investigators, and Other Stakeholders.”
This document contains recommendations, not binding regulations, and is still open for comment from clinical trial experts. But it provides valuable insights into how the FDA thinks about digital health technologies (DHTs) and collecting participant data remotely.
Here are a few frequently asked questions about the FDA’s guidance for any clinical trial organization that wants to use decentralized and hybrid trials.
- What are digital health technologies?
- What are the benefits of digital health technologies?
- What should sponsors or investigators think about when choosing DHTs?
- What information should sponsors put in their regulatory submissions if they’re using DHTs?
- How can sponsors perform verification and validation of DHTs?
- Do trials that use DHTs need different endpoints?
- What risks come with digital health technologies?
- Do sponsors have to mention DHTs in the informed consent form?
- Where should data from DHTs be stored?
- What are sponsors’ duties when using DHTs?
- What are investigators’ duties when using DHTs?
The information presented here is for informational purposes only and is not for implementation in operations. Please consult official ICH E6(R3) guidance documents for operational use.
What are digital health technologies?
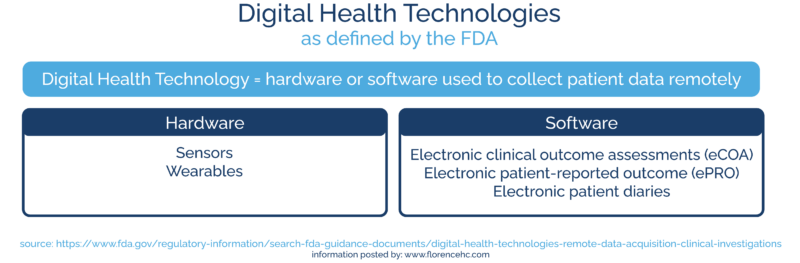
In this guidance, the FDA defines “digital health technologies” as hardware or software used to collect patient data remotely.
Technology that’s only used for monitoring between sites and sponsors would fall under different FDA guidelines (although many forms of technology, Florence included, help with both storage of patient data and communication between sites and sponsors.)
Digital health technologies include:
Hardware
- Sensors
- Wearables
Software
- Electronic clinical outcome assessments (eCOA)
- Electronic patient-reported outcome (ePRO)
- Electronic patient diaries
If you’d like to learn more about the different types of decentralized clinical trial software, you can check out our breakdown here.
These DHTs can be used to collect information from quantitative data (like heart rate) to qualitative data (like participant behavior or emotions.)
Sponsors, sites, and CROs should also note that DHTs aren’t the same as medical devices used for the treatment of a disease. DHTs are used to measure whether a treatment or device is working–they don’t provide any treatment themselves.
If you’d like to learn more about the full range of clinical trial technologies, you can check out our State of Clinical Trial Operations Technology survey.
What are the benefits of digital health technologies?
The main benefits of DHTs are:
- Recording data frequently and in a variety of locations
- Reaching patients who can’t travel to the study site often
- Sending data straight to investigators
DHTs help research sites record data directly from trial participants, wherever they may be.
The FDA provides the example of tracking participants’ heart rates while they’re at home, school, or work. This gives research staff a better understanding of how treatments function in the real world, not just at the study site.
Digital health technologies can also help participants who can’t travel to the study site frequently. Patients who don’t have transportation, can’t take time off work, have chronic illnesses or disabilities, or live hours away from sites are often excluded from clinical trials.
Data captured by DHTs can be transmitted directly to investigators, sponsors, and site staff. This lets clinical trial organizations receive data from participants across countries or even across the world.
Some digital health technologies even include the ability to transmit data while maintaining blinding or masking.
What should sponsors or investigators think about when choosing DHTs?
The FDA recommends that sponsors or investigators take multiple factors into account when deciding which digital health technologies to use:
- The clinical trial’s population
- The design and operation of the DHT hardware or software
- The benefits of providing DHTs vs. letting participants use their own devices

The clinical trial’s population
Investigators or sponsors should think about the languages, age, and technical aptitude of the population they are trying to reach with a clinical trial.
Participants may need in-person visits, extra training, or accessibility features on their DHTs in order to use them effectively. This could include DHTs with large text, buttons instead of a touchscreen (if it’s a wearable), or built-in translations.
Before embracing a form of digital health technology, the FDA encourages sponsors and investigators to explore how the DHT works.
If it’s hardware:
- Is it heavy or clunky for participants?
- Does it have power needs that could cause data disruptions?
- Can it deal with environmental changes, like changes in temperature or exposure to rain?
For both hardware and software, the FDA also urges clinical trial organizations to investigate how much data the software can store and whether it provides notifications to the participant or investigator if the data flow is interrupted. Consistent and accurate data is vital for clinical trials, so investigators need to know immediately if data is no longer being recorded.
Sponsors will also need to determine whether the software includes adequate privacy and security features. Because the clinical trial industry has stricter data privacy standards than the consumer tech industry, many sponsors opt for technology built specifically for clinical trials over general multipurpose tech.
The benefits of providing DHTs vs. letting participants use their own devices
Sponsors or investigators will have to provide DHT hardware, like sensors and wearables, to participants. But DHT software is different. The FDA lets trial designers decide whether to provide participants with smartphones, tablets, and smartwatches or let participants use their own technology.
There are benefits to both approaches. Letting participants use their own devices can make trials easier, since participants won’t have to learn a new piece of tech.
However, sponsors have to ensure that these devices meet technical and performance requirements. If a personal device is out of date, it might not record data accurately and consistently.
Sponsors also have to make sure that they don’t exclude participants from trials because they can’t afford DHTs. A trial that relies on patients having their own smartphone, tablet, or smartwatch will reach a smaller population. When sponsors provide all necessary technology, clinical trials become more inclusive.
For more on what kinds of DHTs sites and sponsors can choose from, check out our State of Clinical Operations Technology Report for 2022.

What information should sponsors put in their regulatory submissions if they’re using DHTs?
The FDA asks sponsors to explain in their regulatory submissions why the DHT they’ve chosen is fit-for-purpose for the clinical trial.
To prove a DHT is fit-for-purpose, sponsors should include the following information in their submissions:
- Relevant physical characteristics of the DHT
- The data the DHT provides to the sponsor and investigator
- How the DHT measures clinical events (ex. using accelerometry to measure steps or photoplethysmography to count heartbeats)
The sponsor must also explain their verification and validation process, the endpoints they’re using, and their risk management plan.
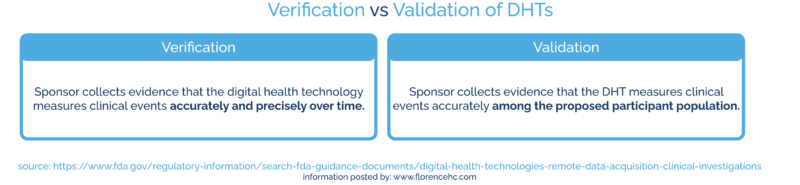
How can sponsors perform verification and validation of DHTs?
During verification, the sponsor collects evidence that the digital health technology measures clinical events (like acceleration, temperature, or pressure) accurately and precisely over time.
During validation, the sponsor collects evidence that the DHT measures clinical events accurately among the proposed participant population.
So if a DHT measures a person’s footsteps accurately over time, it’s verified. But if the sponsor plans to use that DHT with participants who have Parkinson’s disease, they need to test it on people living with Parkinson’s before it’s validated.
Running verification and validation studies
The FDA suggests that the verification and validation process should begin with benchtop studies, move on to testing in healthy volunteers, and end with testing on individuals from the population involved in the trial.
If the DHT vendor or another third party has already performed verification and validation, sponsors can include that data in their regulatory submission. If the data isn’t available yet, the sponsor will need to perform verification and validation themselves.
Verification and validation can also include usability studies. Once they know whether the DHT measures participants’ data accurately, sponsors can hold usability studies to determine whether participants understand how to operate the DHT.
All of this evidence proves that the DHT is reliable to the FDA, which makes the agency more likely to approve the sponsors’ regulatory submissions. You can learn more about the connection between clinical trial software and FDA submissions here.
Do trials that use DHTs need different endpoints?
DHTs typically provide research sites with more data, more frequently. This can affect the endpoints sponsors or investigators choose to focus on.
The sponsor or investigator can determine what endpoints are most critical for their study, as always. But when digital health technologies are involved, sponsors and investigators also need to come up with a statistical analysis plan. This plan explains how they will analyze the data and connect it to the study’s endpoints.
The statistical analysis plan should also account for what happens if there’s a data interruption.
If a software update or transmission failure causes some participant data to go missing, can that participant’s data still be used for the trial? At what point does an error render data unusable? All of these answers belong in the statistical analysis plan.
More data can help clinical trials more accurately reflect participants’ real-world experiences. But to take advantage of this extra data, sponsors need to think carefully about endpoints and statistical analysis plans.

What risks come with digital health technologies?
Digital health technologies come with their own sets of risks that need to be discussed with the IRB and included in informed consent documents. The FDA also recommends creating a risk management plan that’s included in the regulatory submission.
The risks you should address include:
Clinical risks:
- A wearable DHT causing injuries, such as rash or skin irritation
- A wrong measurement from the DHT leading to a wrong treatment (for example, if the DHT is a glucometer, an incorrect reading could lead to too much or too little insulin)
Privacy risks:
- A breach of the DHT that could leak patient information
- A third-party vendor collecting data that they shouldn’t
Some of these risks can be prevented entirely. For example, sponsors can choose DHTs made by a vendor who won’t collect participant data. But for risks that can’t be eliminated, sponsors need to include a risk management plan in their regulatory submission.
If you’d like to learn more about the challenges of using technology and how to overcome them, we’ve included a few tips in our State of Clinical Operations Technology Report.
Do sponsors have to mention DHTs in the informed consent form?
Yes. The informed consent form must contain any reasonably foreseeable risks to the participant. This includes clinical or privacy risks from the DHT.
The informed consent document should also include what information will be collected by the DHT, who can see that information, how long they can see it for, and what they’re allowed to do with it.
Fortunately, sites, sponsors, and IRBs need to tell participants how their data will be used during all clinical trials, not just trials that use DHTs. This means adding in how DHT data will be used is a relatively small change.
Where should data from DHTs be stored?
The FDA says that all relevant data captured by a DHT needs to be stored in a “durable electronic data repository.” The FDA would then consider the data in this repository the source data.
This electronic data repository can take many forms. Some sites and sponsors use an online drive, while others rely on their eISF or eTMF. eISFs and eTMFs can be great places to store source data because they already follow clinical trial compliance regulations.
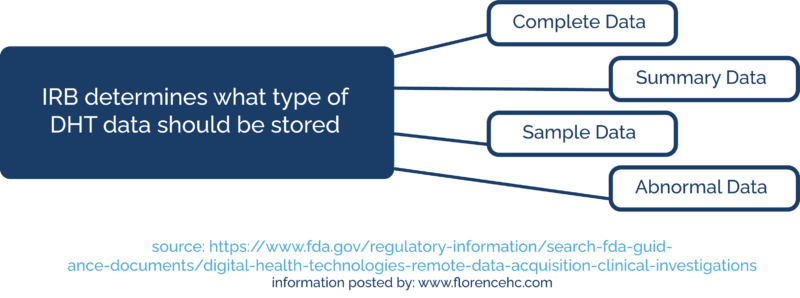
The sponsor can work with their IRB to determine what type of DHT data they should store. The IRB may ask them to store complete data, summary data, sample data, or abnormal data from continuous recording.
Sponsors also must ensure they can store the DHT data for long enough to meet FDA inspection requirements. That’s why they should look for software with a long-term cold storage feature.
What are sponsors’ duties when using DHTs?
When using DHTs, the FDA asks sponsors to:
- Ensure training of trial participants and trial personnel on DHTs
- Develop a plan for technical assistance to trial participants or study personnel
- Develop a risk management plan to address potential problems trial participants may experience (both clinical and privacy)
- Develop a safety monitoring plan (in case a sensor shows a medical crisis)
- Ensure data goes into a durable electronic repository
A good DHT vendor should be willing to help sponsors with all of these challenges, especially employee training, technical assistance, risk management, and data storage.
What are investigators’ duties when using DHTs?
The FDA also provides guidance for what investigators should do when using DHTs:
- Ensure that participants understand what information will be collected by the DHT and how the security and privacy of that data will be maintained
- Train participants on using the DHT according to the protocol
- Review data from the DHTs periodically
Once again, the DHT vendor can help achieve these goals. A good DHT vendor can explain how they ensure data security and privacy and offer training tips and documentation.
How Sponsors Can Prepare to Use Digital Health Technologies
With 92% of hospitals and health systems using decentralized trial methods, digital health technologies are here to stay. That makes the FDA’s stances on DHTs vitally important.
Although the FDA’s DHT guidance hasn’t been finalized, it’s still an invaluable guide for sites, sponsors, and CROs who want to keep up with the hybrid trial movement. And if you’d like to learn more about how your organization can prepare for the future of clinical trials, check out the key findings from our State of Clinical Trial Operations survey, released in January 2022.
