Take Back Your Workflows
Florence’s Site Enablement Platform for Sites
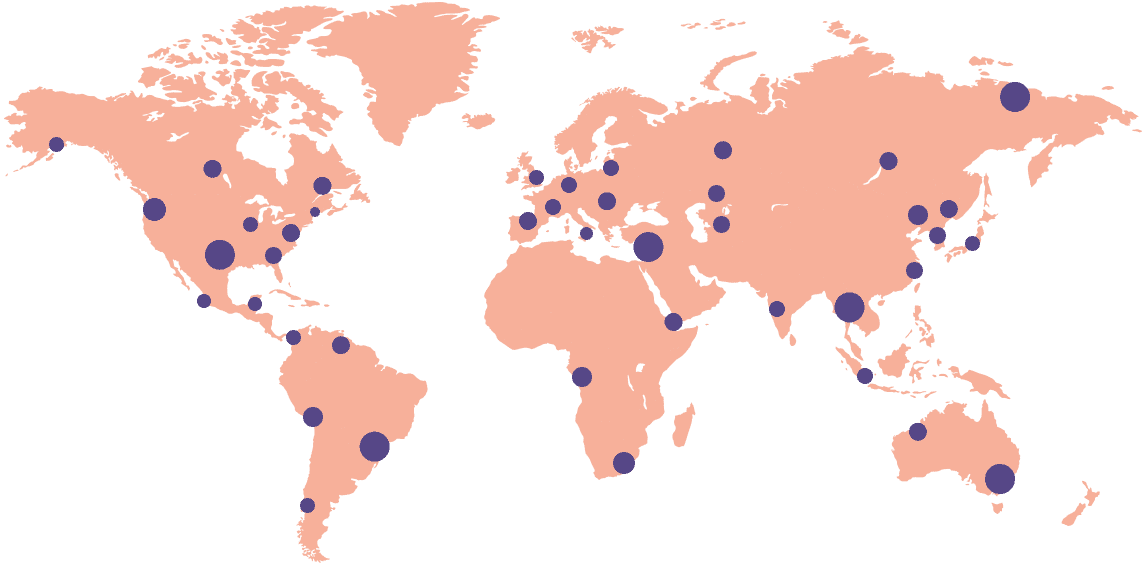
Florence’s solution is tailored made to make the lives of every research site easier. As the platform of choice for 18,000+ research sites across 55+ countries, and rated #1 out of 190 vendors by site users, Florence is dedicated to ensuring your success.
It’s Time To Take Back Your Workflows
Digitize, automate, and integrate your electronic investigator site files, participant binders, and logs.
Go Digital from Start-up to Close-out
Transform your study start-up process with our all-in-one platform. Create, edit, distribute, collect, sign, and review all necessary files electronically, streamlining the process and reducing study start-up times by up to 40%. One NCI Cancer Center reduced their average time to sign from 2 weeks to just 4 hours.
Experience Flexible Workflows
eBinders lets you set up your structures and workflows the way you need while maintaining regulatory compliance. Empower remote workforces to help your team work from anywhere.
Enjoy an Easy-to-use Interface
Florence is rated the #1 clinical trial workflow platform on G2 for ease of use, ease of setup, and customer support. Even the most paper-loving PI will enjoy moving to Florence eBinders – and our industry-leading implementation and customer operations team will help you do it.
Automate Compliance
Built-in compliance features include automated audit trails, version control, extensive user permission options and in-app redaction. Plus, your team is supported by our extensive compliance program to help develop SOPs for going digital.
Enable Remote Monitoring
Give sponsor and CRO monitors secure and compliant access to your electronic investigator site file and redacted participant binder. Automate scheduling of remote visits, track monitor activity, and communicate with your monitor on follow-up items.
Customizable Electronic Logs
Improve patient safety, increase study speed, and ensure data quality with electronic clinical trial logs on Florence. Customize workflows for any log you need – Delegation of Authority Logs, Adverse Event Logs, Training Attestation Logs, Informed Consent Version Logs, Screening/Enrollment Logs, and more.
The Site Enablement Platform
Discover how Florence’s Site Enablement Platform helps sites streamline operations, enable remote monitoring, and integrate study workflows.
SITE START-UPReduce Bottlenecks in Site Start-upDigitally manage the entire study start-up process across a single site or multiple locations. Orchestrate start-up workflows, documents, signatures, and reviews in a single platform. Integrate directly with other systems used in the start-up process (CTMS, IRB, etc.) to eliminate duplicate tasks. |
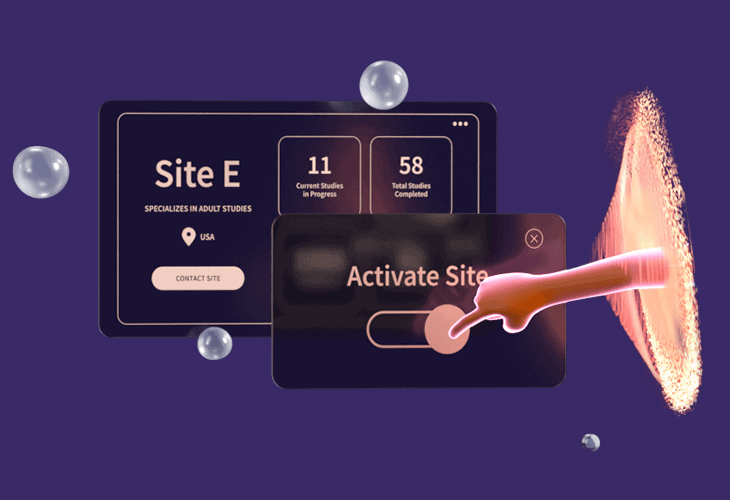 |
SITE CONDUCTSimplify FPI to LPODigitize and integrate every document workflow from first-patient-in to last-patient-out, reducing site burden by up to 40%. Enable your site with the best-in-class eISF (eBinders) and eConsent platform to streamline operations while activating remote monitoring capabilities and advanced performance insights. |
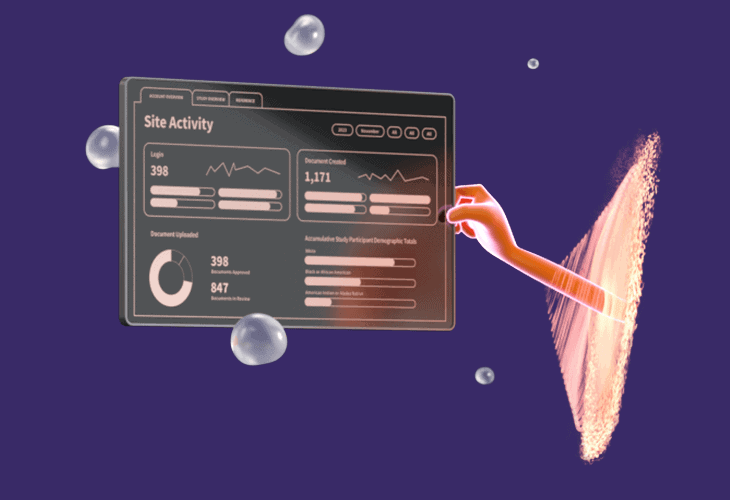 |
SITE CLOSE-OUTSigned, Sealed, SubmittedClose out your studies on time with improved submission quality, compliance, and completeness. Collect and finalize all documents quickly, process through automated QC workflows with your sponsor/CRO, and enable long-time archiving capabilities to maintain inspection readiness after the study closes. |
 |
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
18k+
Sites activated on the platform
55+
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Make Your Teams Happy
(Sponsors will thank you too)
Why being rated #1 out of 190 clinical trial platforms on G2 by research sites matters for you.
Collaborating in real-time on a single document management platform helps us tackle study tasks faster and keep research on track.
Dr. Christina Brennan
VP of Clinical Research

Enable Sites, Accelerate Trials
E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.
Loved by Sites
Trusted by Sponsors