SITE CONDUCT
Simplify FPI to LPO
Digitize and integrate every document workflow from first-patient-in to last-patient-out, reducing site burden by up to 40%.
Enable sites with the best-in-class eISF (eBinders) and eConsent platform to streamline their operations while activating always-on remote monitoring capabilities, advanced site performance insights, and real-time document exchange with the eTMF.
How Florence Streamlines Site Conduct
Enable continuous digital collaboration with every study site for the complete study life-cycle including remote monitoring and remote source data verification and review.
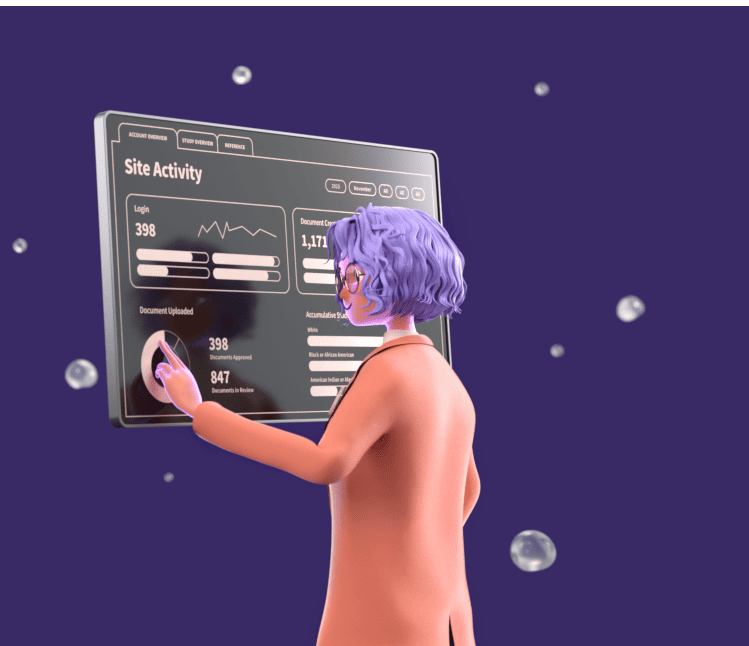
Gain 24/7 Visibility Into Every Site
Because every site maintains their complete eISF on a connected platform you gain real-time visibility into all operations including document status, tasks, and other integrated workflows.
Ensure Site Adoption
Research sites love using Florence, ranking eBinders the #1 platform for ease-of-use and quality of support out of 190 vendors. This means sites adopt the platform (95%+ average adoption) and use it for the end-to-end management of your study, so you never have to worry about disrupting site workflows.
Activate Remote Monitoring
Provide your CRAs with a complete remote monitoring experience that integrates directly with the sites workflow. Most sponsors reduce their on-site visits by over 50%, cutting travel costs, maximizing CRA efficiency, and enabling a continuous monitoring posture.
Enable Remote Source Data Review
A simple front-end integration to the sites existing EMR/EHR or other eSource platform, coupled with easy-to-use paper source capture tools, allows your CRAs to seamlessly conduct source data review and verification remotely without disrupting the site workflows.
Maintain Inspection Readiness
Know the status of every document at every site in real-time across your entire study, while enabling the ability to drill down to a specific document and assign remediation tasks to the site.
Gain Visibility into CRO Operations
When outsourcing monitoring and site management to a CRO Florence’s Site Enablement Platform gives you real-time visibility into their operations so you can ensure you remain a sponsor of choice at your study sites.
Work Seamlessly with Frontier Sites
Working with new to research sites that are geographically dispersed without worry about compliance issues and operational blindspots because you gain 24/7 visibility directly into their operations.
Increase CRA Productivity
Remote monitoring directly integrated with the sites workflows allow most CRAs to monitor and manage 2x more sites than traditional methods, helping your organization scale your studies without increasing staff.
“[With Florence’s SiteLink] we’re providing a more valuable site management experience, while allowing more time and opportunities for site support, compliance reviews and continuous monitoring of patient safety and study quality.”
Rajneesh Patil
VP of Clinical Operations and Head of Digital Strategy
IQVIA

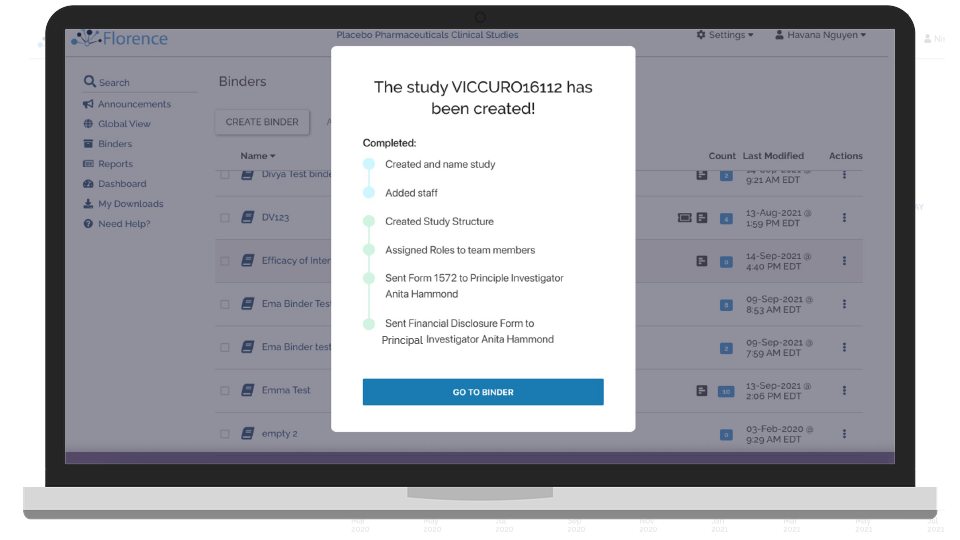
Start-up Sites Remotely
Remotely activate and start-up your study sites with a complete suite of electronic binder solutions.
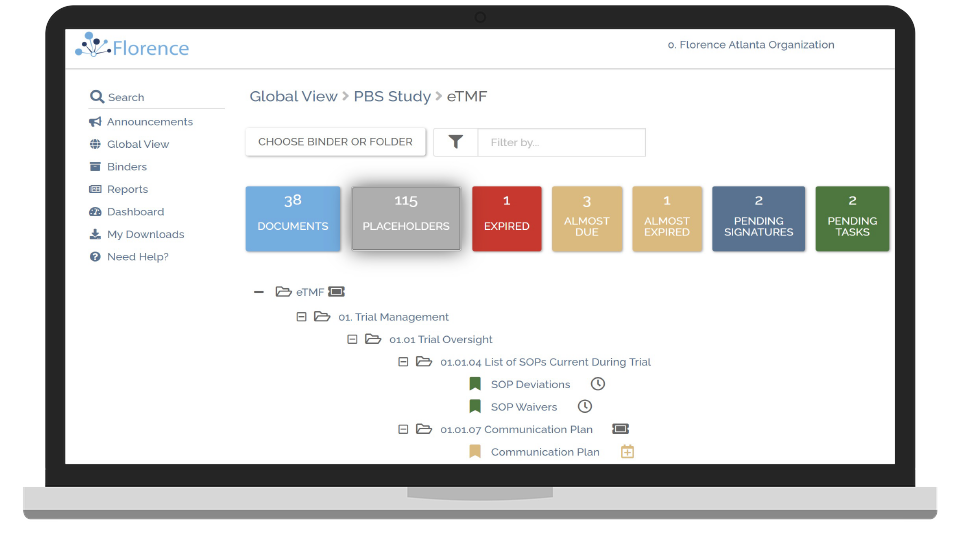
24/7 Continuous Monitoring of Sites
Access your study sites’ Electronic Investigator Site Files anytime for continuous monitoring.
Communicate and Collaborate with Sites Remotely
Assign tasks and send compliant communications directly in the sites’ eISF workflows.
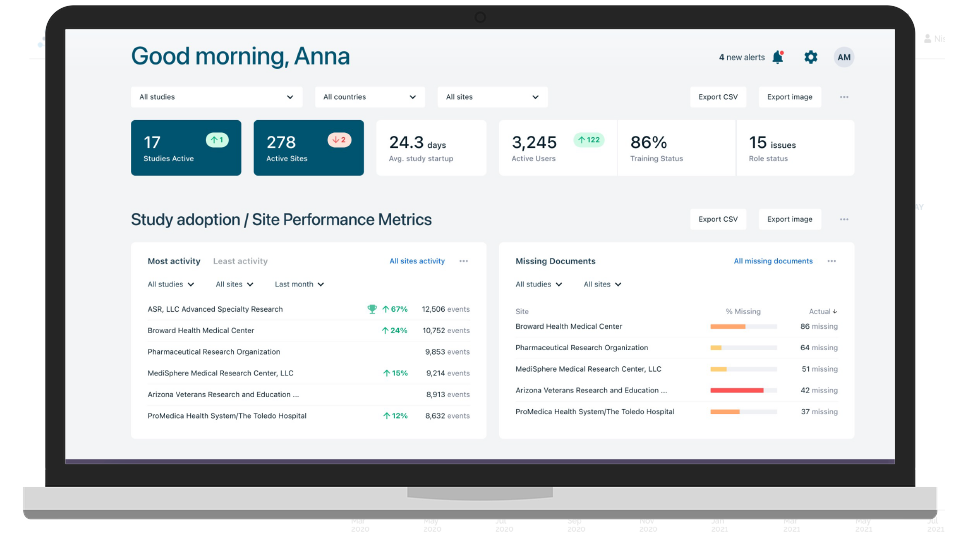
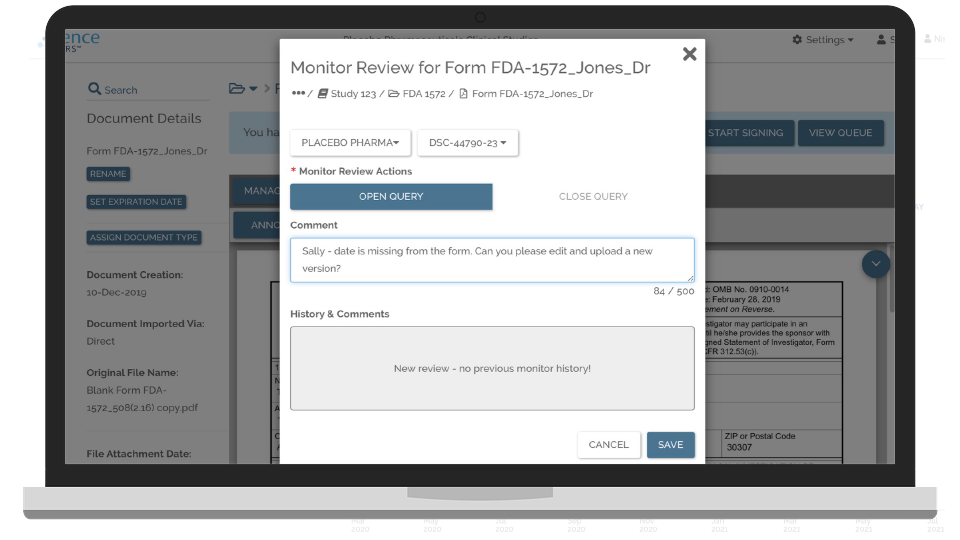
Quality Control and Sync Documents to eTMF
Eliminate the need for mailing, emailing, and faxing study documents and for legacy site upload portals by directly integrating your eTMF with your sites’ eISFs.
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
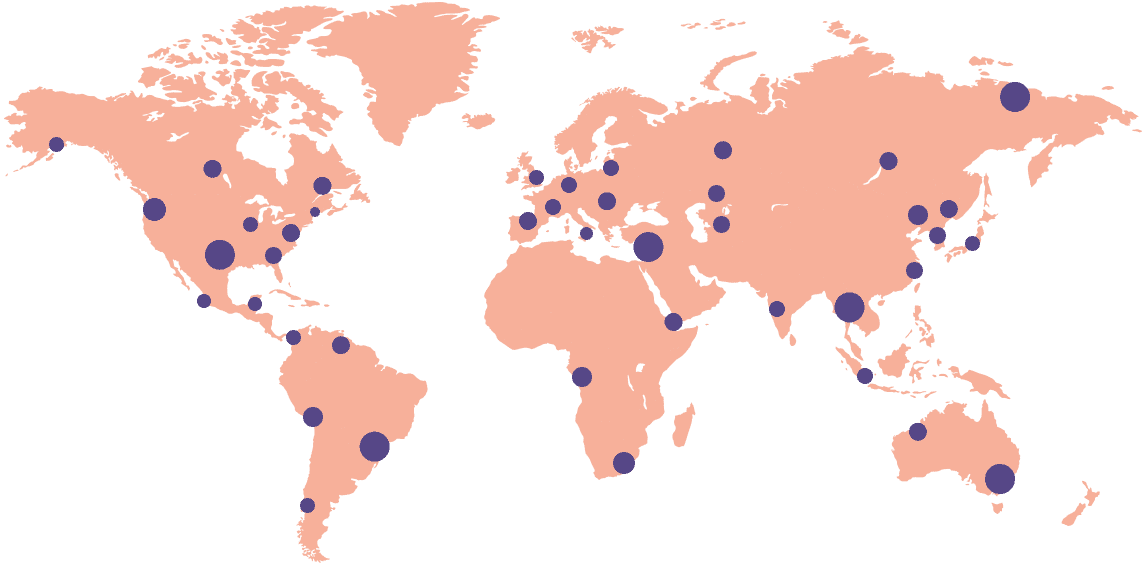
12k
Sites activated on the platform
45
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Trusted by Sponsors,
Loved by Sites

Enable Sites, Accelerate Trials
E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.















