ICH GCP E6(R2) Compliance in Clinical Trials
The ICH GCP E6(R2) guidelines have been amended to encourage implementation of improved approaches to clinical trial design, conduct, oversight, recording and reporting while continuing to ensure human subject protection and reliability of trial results. Standards regarding electronic records and essential documents intended to increase clinical trial quality and efficiency have also been updated.
To help you navigate these standards, we compiled the below information.
We’ve worked with the sponsors, IRBs, and sites to design Florence SiteLink™ as an innovative solution simplifying compliance with the updated ICH GCP E6(R2) standards for remote oversight, recording, reporting, electronic records and essential documents.
What is ICH GCP E6(R2)?
Since the development of the ICH GCP guideline, the scale, complexity, and cost of clinical trials have increased. Evolutions in technology and risk management processes offer new opportunities to increase efficiency and focus on relevant activities.
When the original ICH E6(R1) text was prepared, clinical trials were performed in a largely paper-based process. Advances in use of electronic data recording and reporting facilitate implementation of other approaches. For example, centralized monitoring can now offer a greater advantage, to a broader range of trials than is suggested in the original text.
Therefore, this guideline has been amended to encourage implementation of improved and more efficient approaches to clinical trial design, conduct, oversight, recording and reporting while continuing to ensure human subject protection and reliability of trial results. Standards regarding electronic records and essential documents intended to increase clinical trial quality and efficiency have also been updated.

Don’t simply comply with the new ICH GCP E6(R2) guidelines, harness them to accelerate your clinical trials.
The essential ICH GCP E6 (R2) Implications
The updated ICH GCP E6 (R2) guidelines have significant implications for clinical trial sponsors and sites. Florence has worked with sponsors and sites to develop Florence SiteLink™ to address the following primary requirements of these new guidelines:
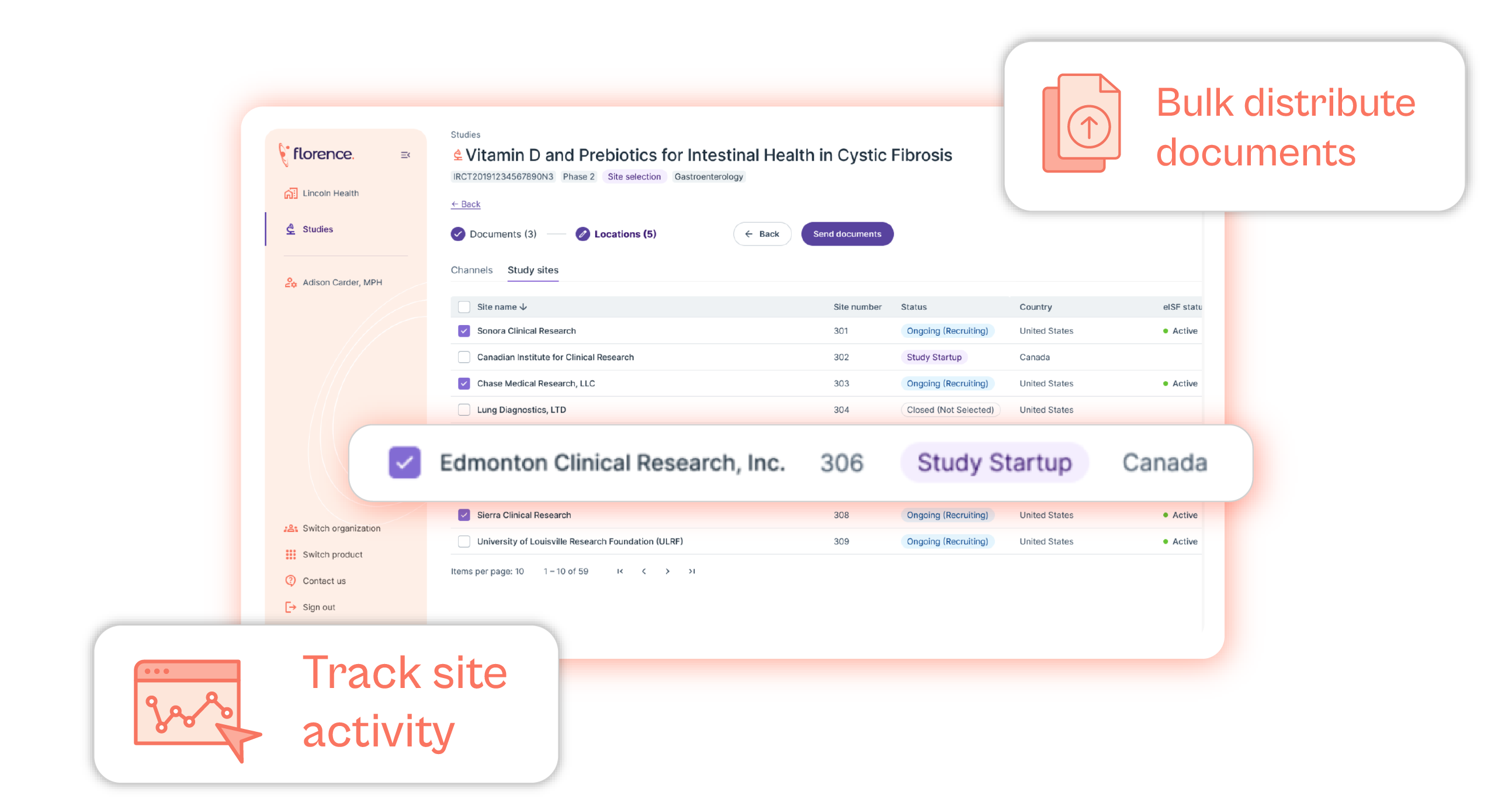
- Remote Oversight and Monitoring (Section 5.18) – Gain real-time access to all of your clinical trial sites and CROs. This remote oversight capability complies with ICH GCP E6(R2) sections 5.18.3, 5.18.4, 5.18.5, 5.18.6, and 5.18.7. Florence SiteLink™ gives sponsors the ability to:
- Centralize monitoring of all sites.
- Analyze site performance metrics.
- Identify missing, expired, or incomplete files.
- Be alerted of protocol deviations.
- Generate immediate remote monitoring reports of all monitor activities.
- Maintain a monitoring plan.
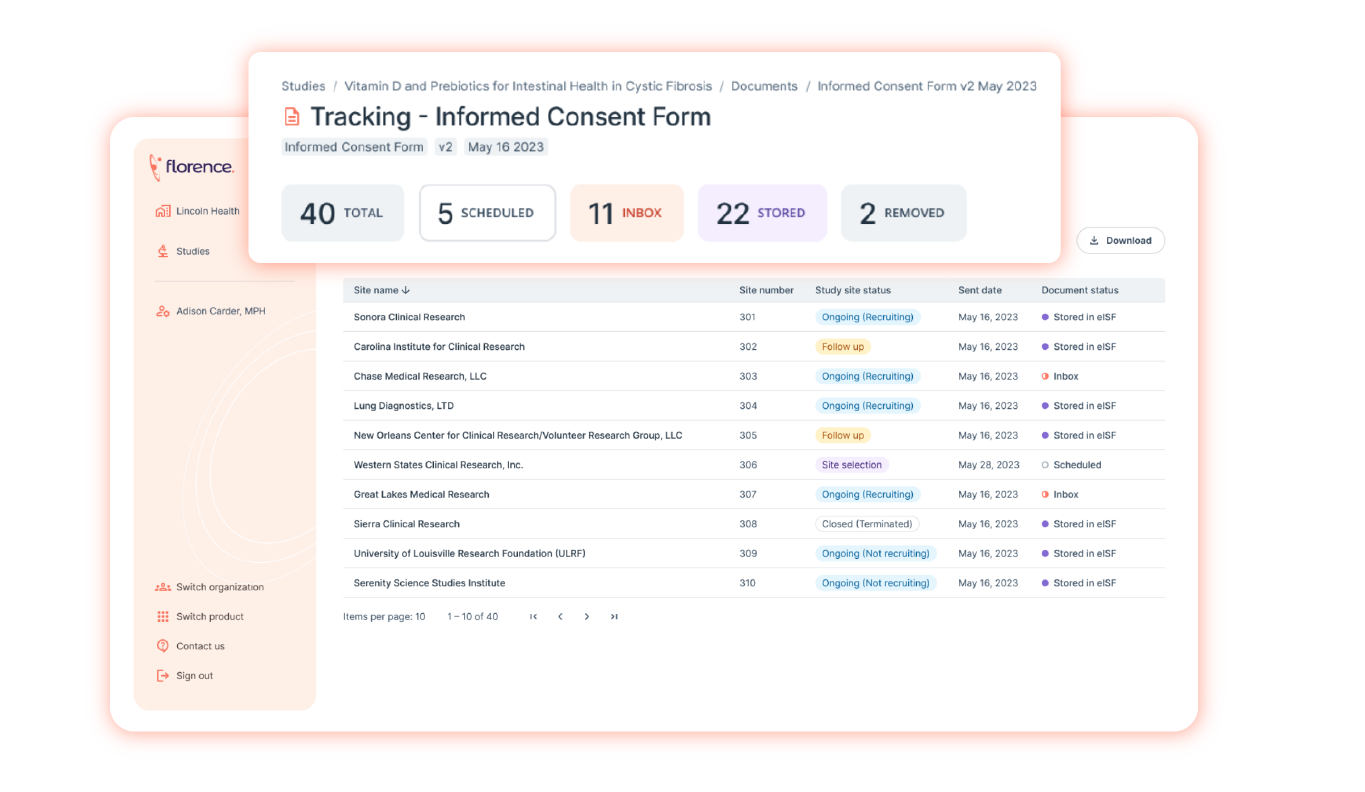
- Centralize Essential Documents (8.1) – Enable centralized storage and access control of all regulatory and source documents in a single-source of truth. This capability complies with ICH GCP E6(R2) section 8.1. Florence SiteLink™ gives sponsors and sites the ability to:
- Maintain a record of the location(s) of their respective essential documents.
- Real-time document identification, version history, search, and retrieval across all stakeholders.
- Electronic certified copies with fully compliant electronic signatures and permission controls.
- Investigator control of all essential documents and records generated by the investigator/institution before, during, and after the trial.
- Audit Trails (4.9.0) – Enable an “always on” audit trail tracking all document/folder interactions. This capability complies with ICH GCP E6(R2) section 4.9.0. Florence SiteLink™ gives sponsors and sites the ability to:
- Maintain adequate and accurate source documents and trial records.
- Ensure source data is attributable, legible, contemporaneous, original, accurate, and complete.
- Enable version control to ensure source data is traceable, does not obscure the original entry, and is explained if necessary.
- Provide full audit trails instantly of every file/folder allowing immediate identification of when documents were uploaded, changed, signed or reviewed, and by whom.
How does Florence simplify compliance with ICH GCP E6(R2) standards?