The Site Enablement Platform for Contract Research Organizations (CROs)
Discover how Florence’s Site Enablement Platform helps CROs offer differentiated study management to sponsors that supports enhanced site operations, strengthened site relationships, increased trust with sponsors, and accelerated clinical trials.
Site Start-up
SITE START-UP
Reduce Bottlenecks in Site Start-up
Offer sponsors accelerated study start-up capabilities by activating sites remotely and equipping sites with digital workflows that integrate with their existing workflows.
Deploy the #1 rated eISF platform to sites to enable study start-up document routing and real-time collaboration in just a few clicks. Monitor performance and activity across all sites in a single location. Enable visibility to sponsor partners into site start-up processes to increase trust.
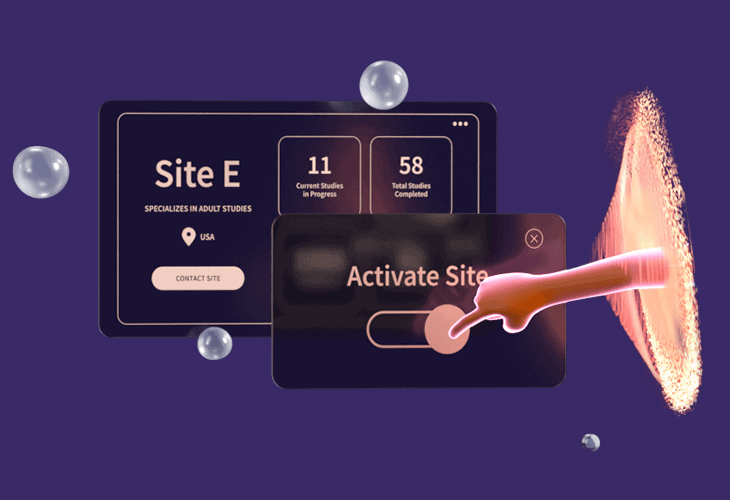
How Florence Streamlines Site Start-up
Start your study process on the workflow platform that digitizes the site-sponsor connection across 18,000+ sites in 55 countries.
Distribute Start-up Documents Fast
Distribute up-to-date start-up documents to every site at once in a central platform and have it appear in their existing workflows if they’re already connected to the Florence network (18,000+ in 55 countries are today).
Rapidly Activate New Sites on Platform
For sites not yet connected to the Florence platform, easily get them set-up and connected with their own fully functioning eISF in a few days with our expert Site Activation Teams that average 95%+ adoption rates and rank #1 in ease-of-setup across 190 vendors.
Increase Trust with Sponsors
Boost trust and transparency with sponsors by offering real-time insights into clinical trial site start-up processes. This innovative solution fosters collaboration and streamlines communication, ensuring efficient trials and increased sponsor confidence.
Monitor Start-up Status in Real-time
See the status of every document at every site in real-time. Has it been viewed, reviewed, filled out, signed? Has the site team completed open tasks for the document? Has the team uploaded the latest CV? Global dashboards let you see across all your sites while giving you the ability to drill down to a specific document at a specific site.
Manage a Global Network of Sites Compliantly
Florence’s solutions are already used in 55 countries around the globe, making it easy for you to centralize and monitor all start-up even if you’re using a CRO or local start-up teams.
Seamlessly Transition to FPI Processes
Because the site continues managing all their regulatory binders, participant binders, logs, and consent in the same platform, you’re able to seamlessly continue to manage end-to-end site operations in one location.
Design Study Structure
Design Electronic Investigator Site File, Electronic Participant Binders, Electronic Logs and start-up workflows to distribute to sites.
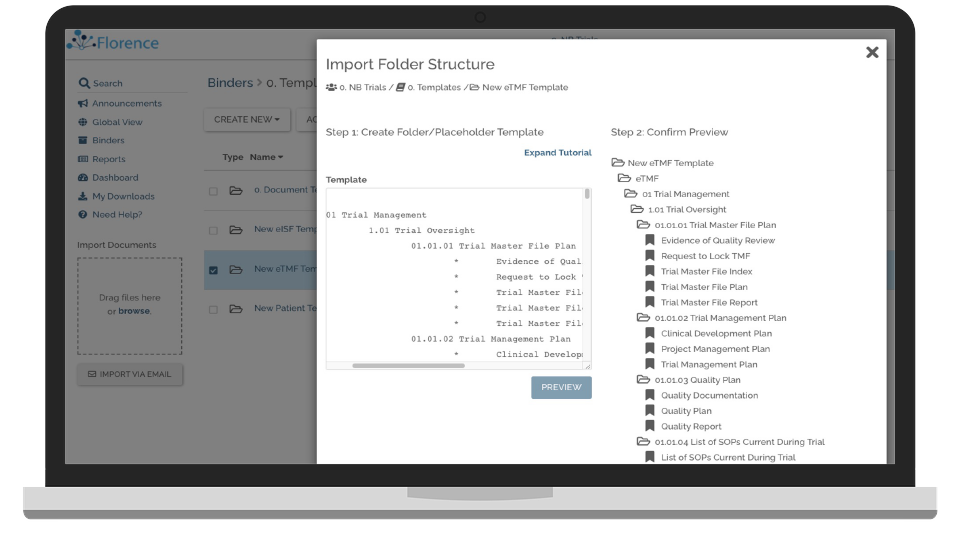
Deploy Electronic Binders to Study Sites
Easily deploy your binder structures, workflows, templates, tasks and logs across study sites in a single platform.
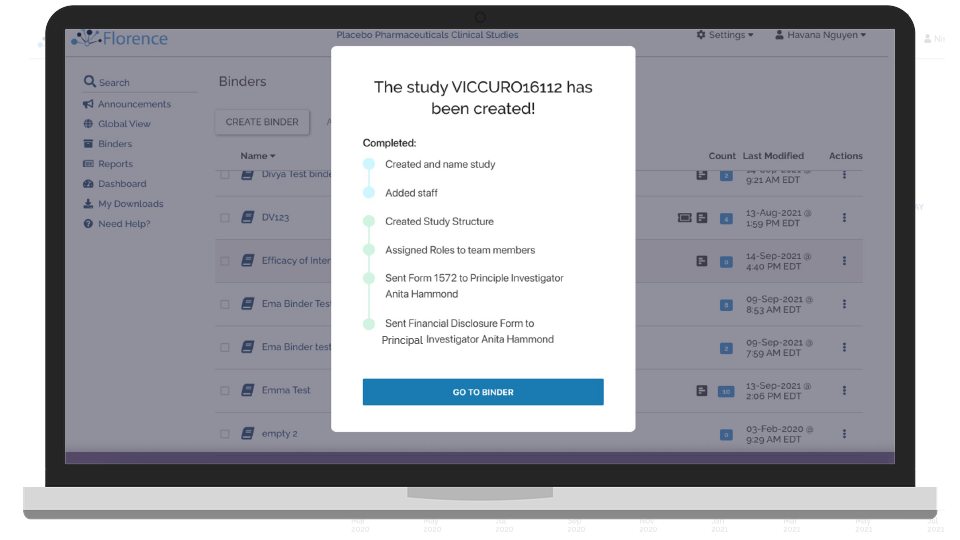
Track Study Start-up in Real-Time
Keep track of every site in your study and their activation and start-up progress.
“[With Florence’s SiteLink] we’re providing a more valuable site management experience, while allowing more time and opportunities for site support, compliance reviews and continuous monitoring of patient safety and study quality.”
Rajneesh Patil
VP of Clinical Operations and Head of Digital Strategy
IQVIA

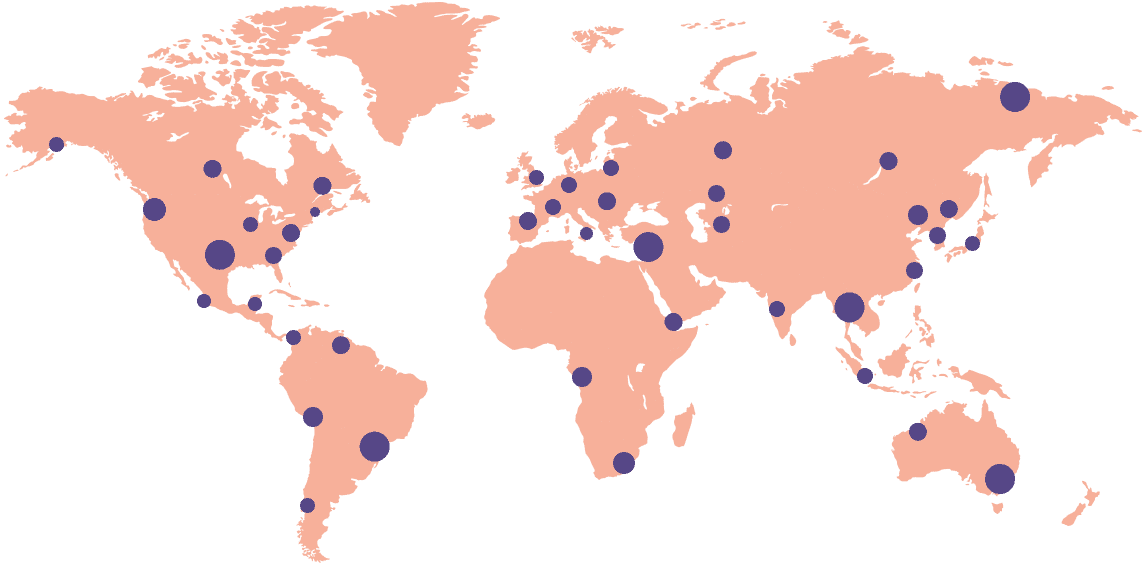
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
18k+
Sites activated on the platform
55+
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Trusted by Sponsors,
Loved by Sites

Enable Sites, Accelerate Trials
E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.









