How the Right Software Can Help Sites and Sponsors Navigate the FDA Approval Process
Sponsors, CROs, and research sites in the U.S. have always understood the importance of the FDA approval process. But with the arrival of COVID-19 vaccines, the general public has started paying closer attention to the FDA’s actions.
All of this attention makes transparent communication between sites, sponsors, and the FDA more important than ever. Research sites need to update source data and regulatory documents quickly to ensure they’re accurate. Then, sponsors and CROs need to instantly access those updates so they can compile accurate documentation for the FDA.
Clinical trial software can help sites, sponsors, and CROs organize the data and documents they need for FDA approvals. However, that software has to be secure, private, and reliable. Here’s how the right software can help you prepare for the difficult and important FDA approval process.
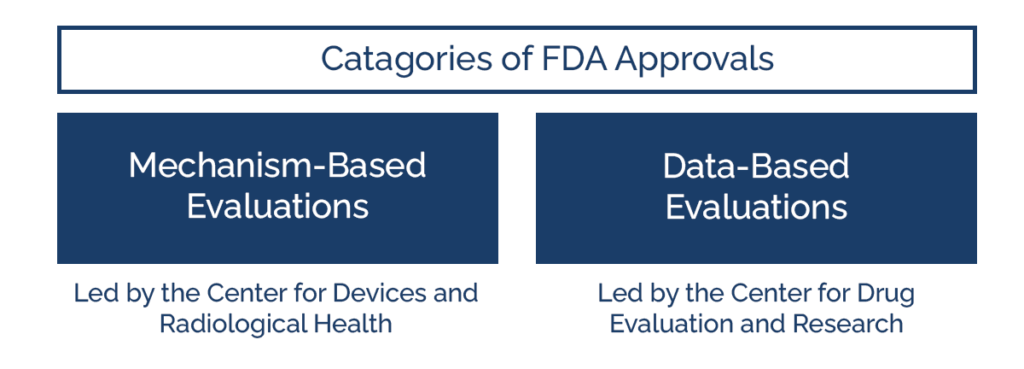
Mechanism-Based and Data-Based FDA Approvals
Dr. Michael Kassin, an Interventional Radiologist at the National Institutes of Health and one of Florence’s founders, separates the FDA approval process into two large categories: mechanism-based and data-based.
Mechanism-based evaluations, led by the Center for Devices and Radiological Health, often require less documentation than data-based evaluations. Dr. Kassin stresses that the CDRH mainly wants to see proof that the mechanism or medical device works.
Data-based evaluations, led by the Center for Drug Evaluation and Research, require a much larger amount of data. New Drug Applications for the CDER often run for hundreds of pages and include data from multiple clinical trials and thousands of patients.
How Can Tech Help with Mechanism-Based or Data-Based Approvals?
Keeping your documents organized is critical for mechanism-based and data-based FDA evaluations. But data-based evaluations require so many documents that compiling them can turn into a full-time job.
Clinical trial software may help with document organization. With the right software, clinical research staff can store and update all of their study documents inside a secure online portal. They can then give sponsors and CROs permission to log in and view the documents that are relevant to them.
Sponsors and CROs will have a much easier time preparing their documents for the FDA if all of the data they need is updated and stored in one location. Sponsors won’t have to wait for sites to email or fax them their latest data, and sites won’t have to worry about whether sponsors have all the information they need for their FDA approval process.
Managing Communication Between Sponsors and the FDA
The FDA encourages sponsors, CROs, and biotech firms to ask them for guidance as they move through their clinical trials. The FDA is even willing to review Investigational New Drug Applications and let sponsors know if their trials are well-designed and likely to produce all of the data they need.
Dr. Richard Pazdur, a physician for the FDA’s Oncology Center of Excellence, told the ASCO Post that the FDA has “a dynamic regulatory environment” with “lots of back and forth with sponsors.” This communication is helpful, but it also means that sponsors have to send documents to the FDA even before they fill out their New Drug Application.
Emailing sites to ask for their latest data or documents, waiting for them to arrive, and then emailing them to the FDA puts a huge burden on already-busy sponsors and CROs. But clinical trial software can help sponsors and CROs with the FDA approval process.
With digital clinical trial software, sponsors can log into the portal they share with their sites and download the documents they need. They can then submit those documents through the FDA’s electronic system for the agency’s review and feedback.
Protecting Patient Data During the FDA Approval Process
Secure online portals can also help you protect patient health information during the FDA approval process. The FDA emphasizes that, though they need access to participant medical records, they don’t typically need participants’ names and only request them in extraordinary circumstances.
The FDA’s guidelines give the principal investigator the responsibility of maintaining the privacy of participant data. However, with data passing between PIs, research site staff, monitors, and the FDA, there are many moments where private health information could accidentally be revealed.
This means that research sites need a reliable way to redact patient information without erasing the information entirely. Though technology isn’t the only way to do this, it is one of the easiest and fastest.
Clinical trial software that is compliant with FDA CFR 21 Part 11 and HIPAA will include the ability to control whether information is redacted or revealed based on a person’s role and their permissions. Sponsors can also control whether private participant information is visible when they send the documents to the FDA.
Faster Approvals from the FDA
The FDA offers streamlined approval programs for drugs or devices that treat serious conditions, especially if those conditions don’t currently have effective therapies. These drugs still have to be examined for efficacy and safety, but the FDA approves them more quickly so critically ill patients can access the therapy.
A study published in JAMA Network Open shows that 64% of FDA-approved medications now go through a shortened approval process. 27% of those drugs were designed for cancer treatment, and many oncology professionals favor the accelerated process.
Dr. Shanthi Ganeshan, who has served as the VP of Global Regulatory Affairs, Oncology for many pharmaceutical companies, praises faster FDA approval processes for being streamlined and getting treatments to cancer patients faster. However, the FDA still needs to ensure the therapies are safe and do what is promised. That’s where data and documentation come in.
How Technology Helps with Streamlining Approvals
When sponsors go through a streamlined approval process, they must communicate with the FDA even more than they do during regular approvals. The FDA provides extensive guidance for the accelerated drug development process and frequently asks for documents or data.
An accelerated FDA approval process makes having updated data and being able to transmit that data more essential than ever. If sponsors and CROs send outdated documents to the FDA or have to wait for their research sites to send over accurate data, the approval process that should be streamlined can instead become slow and clunky.
Building Trust in Technology
Software can help clinical trial sponsors communicate with the FDA more effectively, but that can’t come at the cost of maintaining privacy, security, and compliance. As Dr. Kassin pointed out in his discussion of the FDA approval process, sponsors put a decade of work into nearly every drug they produce.
Trust is therefore paramount: sponsors and research sites need to know that their software provider will protect their data, remain compliant with FDA, HIPAA, and privacy regulations, and not let their documents or data be deleted. When searching for technology, sites and sponsors should ask vendors whether they will ever sell or monetize trial data, how they manage compliance concerns, and whether they have cloud-based back-ups.
Good software vendors should not only be willing to answer these questions: they should be eager to. The right vendor must work alongside sites and sponsors as a partner throughout the entire drug development and FDA approval process.
How Technology Can Help with the FDA Approval Process
Clinical trial software can help sponsors with the FDA approval process by ensuring they have instant access to documents and data from their sites. But given how complex and important FDA approvals are, sponsors and sites can’t afford to settle for any software that comes along. They must search for software that was designed with FDA regulations in mind and that will treat their data with as much care as they do.
If you’d like to learn more about finding FDA-compliant software for your clinical trials, check out our Library of FDA Guidance for Electronic Document Management.

