What do patients think about decentralized clinical trials?
Research sites and sponsors can’t stop talking about decentralized clinical trials (DCTs). But we often forget to ask a vital question: how do patients feel about them?
As of 2021, 95% of research sites use at least one form of decentralized technology, and almost every clinical trial conference features multiple sessions about decentralized, hybrid, or virtual trials.
But DCTs only work if patients want to join them. One study showed that 92% of people want clinical trials to embrace innovative technology. However, patients still worry about technology learning curves or losing their connection with physicians.
For decentralized clinical trials to become a reality, research professionals need to put participants’ experiences and safety first.
Check out these thoughts from patients that can help research sites complete recruitment quickly and run effective hybrid trials.
Most patients like the idea of decentralized trials
Decentralized clinical trials can reach patients who live outside of major cities, which could lead to faster recruitment and greater patient diversity. DCTs can also save participants time and money by not requiring patients to visit the research site for every check-in.
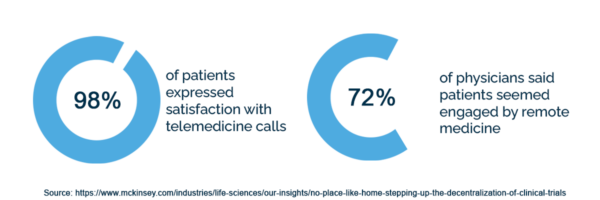
Many patients appreciate the ease of decentralized trial methods. For example, 98% of patients expressed satisfaction with telemedicine calls, and 72% of physicians said patients seemed engaged by remote medicine.
Participants often choose decentralized trials over traditional ones
A study that focused on patients with lower back pain showed that 78% of patients preferred a decentralized clinical trial to a traditional one.
Another promising statistic: 89% of patients who chose the decentralized method completed the study, versus only 60% of patients who chose the traditional method.
This study used a small sample size, but its findings align with what other researchers have heard from patients. After conducting patient focus groups, Deloitte heard that most patients valued having fewer visits to the doctor’s office.
Hybrid trials work well for patients with chronic illnesses
In a survey of patients living with rare diseases (hemophilia, idiopathic pulmonary fibrosis (IPF), myasthenia gravis (MG), and sickle cell disease), 57% of those patients chose hybrid trials over purely virtual or purely in-person ones.
The hybrid method combined check-ins at home, appointments with their local physician, and trips to the research site.
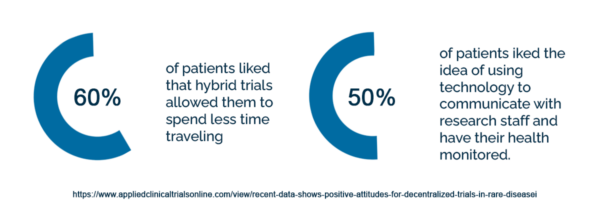
60% of patients liked that hybrid trials allowed them to spend less time traveling, and 50% liked the idea of using technology to communicate with research staff and have their health monitored.
These numbers indicate that many patients with rare diseases value the convenience that decentralized clinical trials offer.
Still, some patients have concerns about using unfamiliar technology and interacting with physicians less. The clinical trial industry needs to proactively address these concerns if they want patients to fully engage with hybrid trials.
Patients have concerns about unfamiliar technology
A McKinsey survey discovered that some patients hesitate to use unfamiliar technology for clinical trials.
Many people have never worked with Electronic Data Capture (eDC), Electronic Patient-Reported Outcome (ePRO), or similar software programs. The experience of learning new technology while learning about the protocols of a trial can overwhelm them.
Ken Getz, Deputy Director of the Tufts Center for the Study of Drug Development, also notes that patients value their relationship with investigators and clinical research staff. Physicians and staff may find it hard to cultivate this relationship when they see patients less often.
The good news: hybrid trials can address both these concerns by focusing on patient education and flexibility.
Comfort with technology varies greatly among patients
Some decentralized clinical trials use very little patient-facing technology. Patients simply visit pharmacies or local doctors’ offices for check-ins instead of going to a hospital or academic medical center.
But most decentralized trials incorporate some technology, whether that means electronic informed consent (eConsent), wearables, or electronic patient diaries. And patients vary greatly in their comfort with that technology.
When patients opt for a traditional trial in place of a hybrid one, their concerns about technology are the number one reason.
For example, one study about overactive bladder conditions that tried to go fully remote couldn’t recruit enough patients and had to be stopped, partially because patients didn’t understand the software program they were supposed to use to record their results.
But that study took place in 2011, when technology was less common, and mainly targeted older participants. More recent studies have seen far greater success.
Patients are becoming more open to clinical trial technology
In a 2018 study of patients with lower back pain, 100% of patients who chose a decentralized trial over a standard one reported satisfaction with the trial’s eICF, eDiary, and telemedicine visits. Patients were less satisfied with the trial’s wearable patch–but they complained that it didn’t adhere correctly, not that they didn’t understand the tech.
The decentralized trial also recruited more diverse patients and patients from rural areas than the standard trial did. While the fully in-person trial recruited an average of 1.25 patients per month, the decentralized trial recruited 4.5 patients per month.
And, of course, 2020 and 2021 saw arguably the greatest triumph of decentralized clinical trials. Pfizer, Moderna, Astra-Zeneca, and Jannsen ran decentralized trials to test their groundbreaking COVID-19 vaccines.
The past few years indicate that patients are becoming more comfortable with decentralized trial technology and that decentralized trials often work. But some patients still have concerns about hybrid trials. How can we make them more comfortable?

Participants need accessible and patient-centric technology
Patient-centric trial design means listening to what participants want from a clinical trial. Research sites could give patients technology training and support in person, through in-app videos and chat, or through telehealth calls.
Sponsors and CROs could also tailor user interfaces to specific patients. An app could come in multiple languages or offer accessibility features like larger fonts and screen readers.
Finally, a decentralized or hybrid trial might offer patients the choice to use very little technology or no technology at all.
Decentralized trials aren’t the same as virtual trials: some hybrid trials use technology, but others have patients visit local clinics and pharmacies instead of just the research site. Hybrid trials can also encourage patients to mix easy technology, like telehealth calls or apps, with in-person visits to a physician or an academic medical center.
When sponsors and CROs design decentralized trials, they need to focus on what mix of technology and in-person visits will work for the patients they want to recruit.
Patients crave a connection with research staff during clinical trials
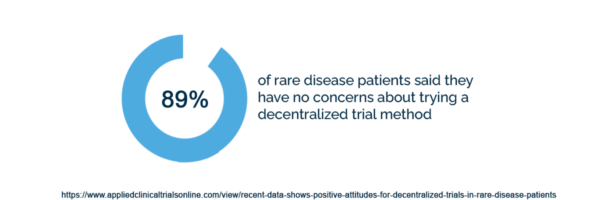
89% of rare disease patients said they have no concerns about trying a decentralized trial method.
But some of the patients who had concerns talked about having adverse reactions while at home. Other patients worried about not understanding the trial materials and what testing they were undergoing.
Whether sponsors and CROs opt for virtual, hybrid, or fully site-based trials, they need to make sure patients feel a connection with the physicians and clinical research staff involved in their treatment.
Patients should know exactly which physician or nurse to contact if they have an adverse reaction. They should also feel confident that their doctor or nurse will be able to advise them and answer their questions.
Sponsors and CROs can build these connections by making sure patients always have a research site to communicate with in person or via telehealth calls. Research sites have knowledgeable professionals on staff who can keep patients informed and make in-person appointments when participants need them.
Participants want to learn from research site staff
Research sites can also provide patient education. Dawn Anderson, the managing director of life sciences for Deloitte, said that patients revealed in focus groups that they crave information on the treatment they’re undergoing and the clinical trial’s results.
“They really want to learn more about their disease, about the testing that is happening,” Anderson told BioPharma-Reporter.
Anderson also notes that, though clinical researchers shouldn’t overwhelm patients with highly technical information, “there’s a wealth of information that can be shared with them to make them feel more like they are a partner in the study.”
Feeling a strong connection with research staff and receiving education from them can help patients become more secure about decentralized clinical trials. But patients also need to understand that decentralized trials are highly regulated and safe.
Patients need information about decentralized clinical trial safety
Decentralized clinical trials must abide by all of the same regulatory standards as traditional trials, but patients don’t always know that.
It’s important that clinical research professionals explain to patients what protocols are in place to protect them. These protocols include regulations around informed consent, eligibility, and risk management.
Decentralized clinical trials must educate patients about informed consent
Most Institutional Review Boards now accept eConsent. But informed consent for decentralized trials goes beyond filling out the consent form on a computer or tablet. Sites need to make sure that patients fully understand what they’re consenting to and can ask questions.
Some eConsent platforms fulfill this need with interactive videos or quizzes. But patients who have trouble understanding these features or find them overwhelming might prefer in-person or telehealth meetings with research staff.
Decentralized clinical trials provide flexible options so patients can complete consent forms in a way that’s easy for them.
Research sites and sponsors must minimize patient risk
Decentralized clinical trials can use a mix of in-person appointments and remote analysis to determine whether patients are eligible for trials. But no matter how sites decide to determine eligibility, they need to make sure they’re following all regulations to minimize the risk for patients.
Clinical research staff also need to educate patients about the eligibility process and what safeguards are in place to protect participants.
No clinical trial is risk-free. But patients may see a clinical trial as more dangerous than it is if research professionals don’t clearly explain safety regulations and the eligibility process to them.
How patients feel about decentralized clinical trials
More than half of patients opt for decentralized clinical trials over fully centralized ones when given a choice. But to make hybrid trials widespread, clinical research professionals need to listen to patients’ concerns about working with technology, connecting with research staff, and protecting their own safety.
Clinical trial teams then need to craft patient-centric trials that ensure participants have plenty of chances to receive support and training, ask questions, and learn about safety regulations.
With support from physicians, nurses, and clinical research coordinators, patients can experience hybrid trials that make them feel safe and valued–and research sites can find the diverse patients they need to run successful clinical trials.
To learn more about making patient-centric decentralized trials a reality, check out our complete guide to Decentralized Clinical Trials for Research Sites, a free download for all clinical trial professionals.

