“Florence’s [eISF + Remote Site Access] platform is helping us to respond to the changing environment due to COVID-19 and further progress COVID-19 research with the capability to perform remote monitoring where approved by regulatory authorities and ethics committees.”
Rob Goodwin
Vice President and Head of the Operations Center of Excellence in Global Product Development
Pfizer

Why Florence? We are already installed at over 8,000 study sites globally.
The network of research sites managing their eISF and source documents on our platform includes major institutions like Northwell Health, Mt. Sinai, Vanderbilt-Ingram Cancer Center, University of Utah, City of Hope, Fred Hutchinson Cancer Research Center, Moffitt Cancer Center, AdventHealth, Atrium Health, and US Oncology, along with thousands of independent sites and site networks like Elligo, Maryland Oncology, Javara, and Tampa General Hospital.
And the best part? If one of your study sites is not already on our platform, we do the hard work for you. You simply send us their info and we will implement, train, and onboard your site so they are ready for your remote powered study.
Connect with the eISF + Remote Site Access Experts
Collaborate with Sites on a Platform they Love
The most important question when deploying technology to collaborate with research sites is “will they adopt it, use it, and love it?“. Florence is the #1 Electronic Investigator Site File (eISF) in the industry installed at more than 7,200 Investigator sites in 26 countries. When you start your next study with Florence you gain access to these sites. In addition, we partner with you to rapidly turn-on your other sites not yet connected to Florence in as little as three days (read the case study here.)
Accelerate Study Startup
Accelerate startup with remote study start-up capabilities by as much as 40% with single-click eReg packet deployment, document status tracker, and the ability to assign and monitor site tasks.

Turn-on Remote Monitoring and Remote SDV
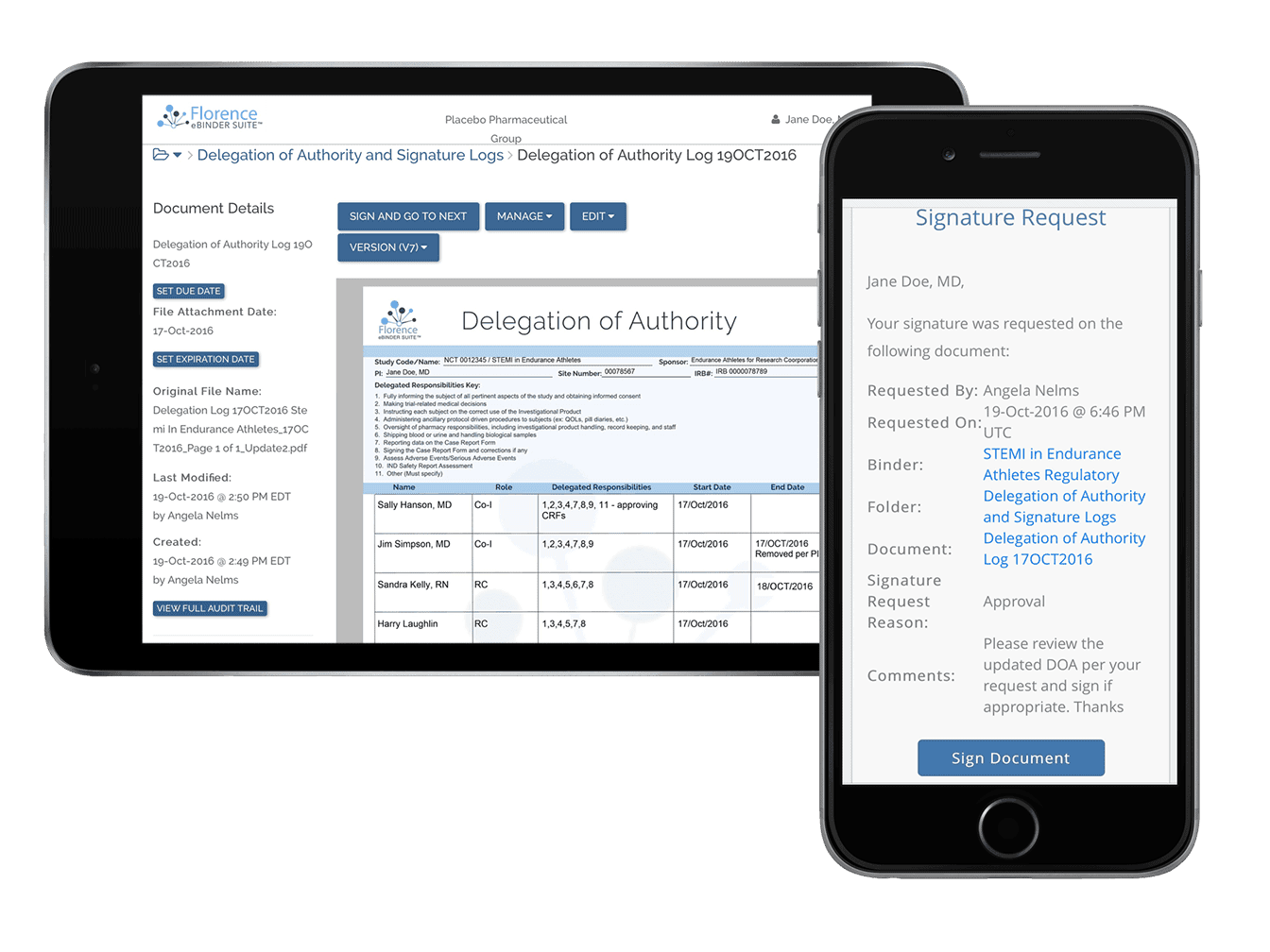
On the Florence platform, Research Sites electronically collect, store, route, and manage source documents.
Site staff collect source documents from any platform (EMR, EHR, eSource, or Paper Source), auto-file it into the appropriate location, and redact sensitive PPI in-app a flexible front-end application.
Sponsors and CROs then access this source data remotely, and securely, in a compliant platform.
The patented permission and access control system powering Florence eHub ensures the research site maintains control and ownership of their document per ICH GCP E6 R2 regulations.
This remote SDV capability gives Sponsors + CROs the ability to access source data and verify CRF and EDC information anytime, anywhere.
Intuitive and Flexible Workspace that Speeds Set-up
Every day matters when starting a new study. Being first-to-market is essential for maximizing revenue potential and delivering life-saving cures to those who need them most. Florence eHub is designed from the ground-up to be intuitive for teams to quickly begin using with minimal overhead.
We Know Sites
We’ve onboarded over 7,200 research sites. We know how to get your sites on the platform and up-to-speed fast, for most sites around the globe in as little as three days.
Power your day with Active Dashboards and Reports
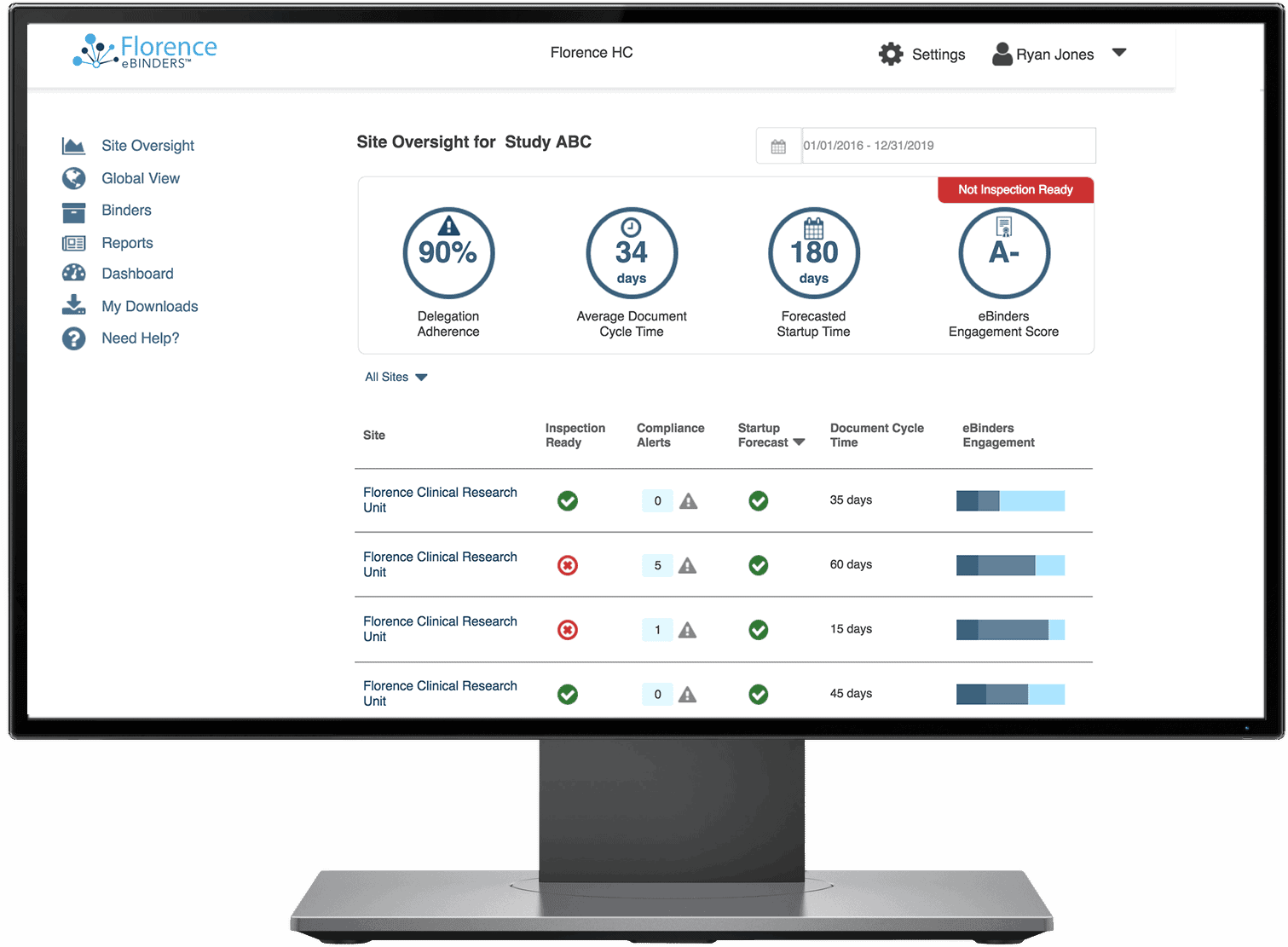
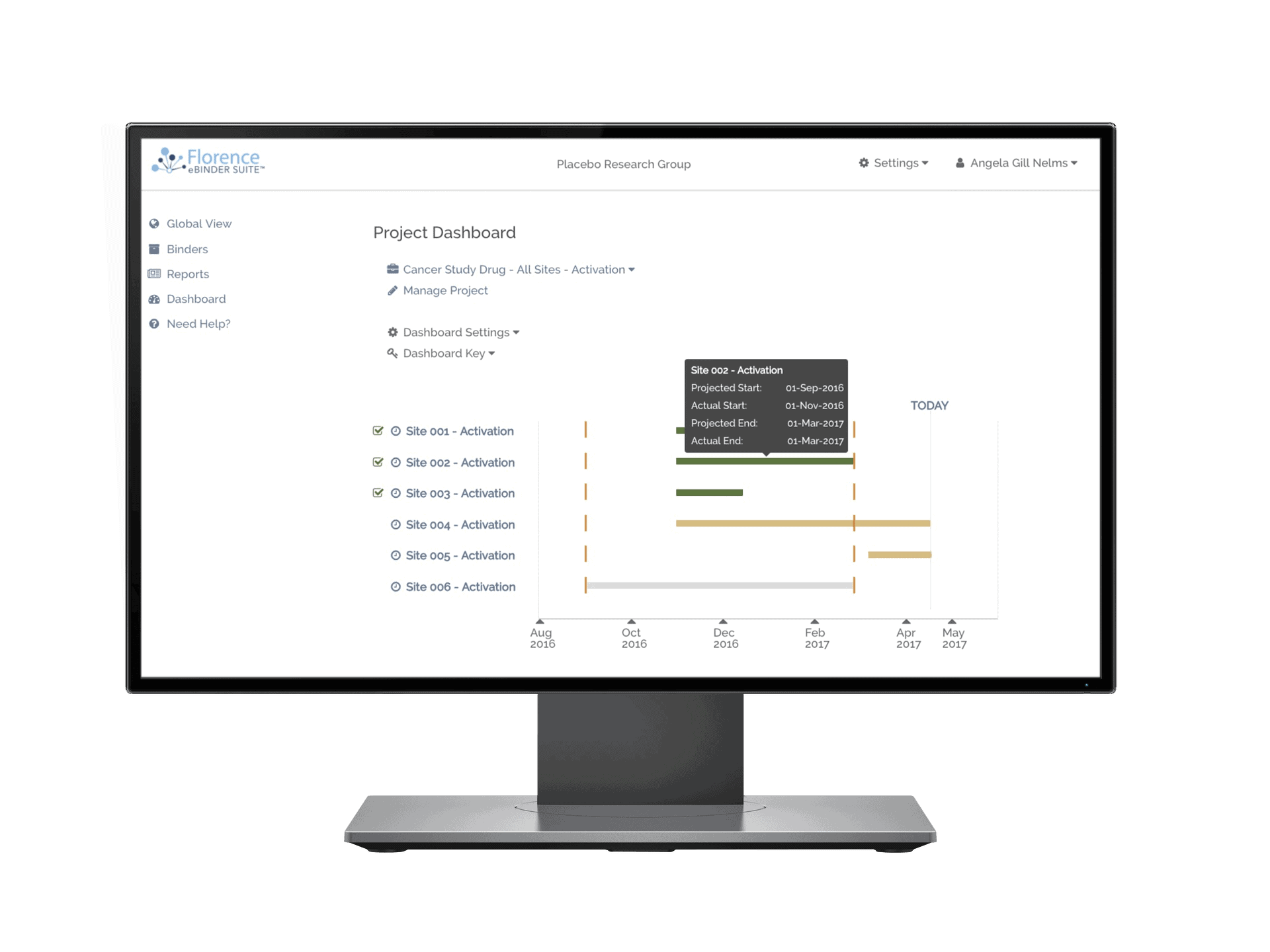
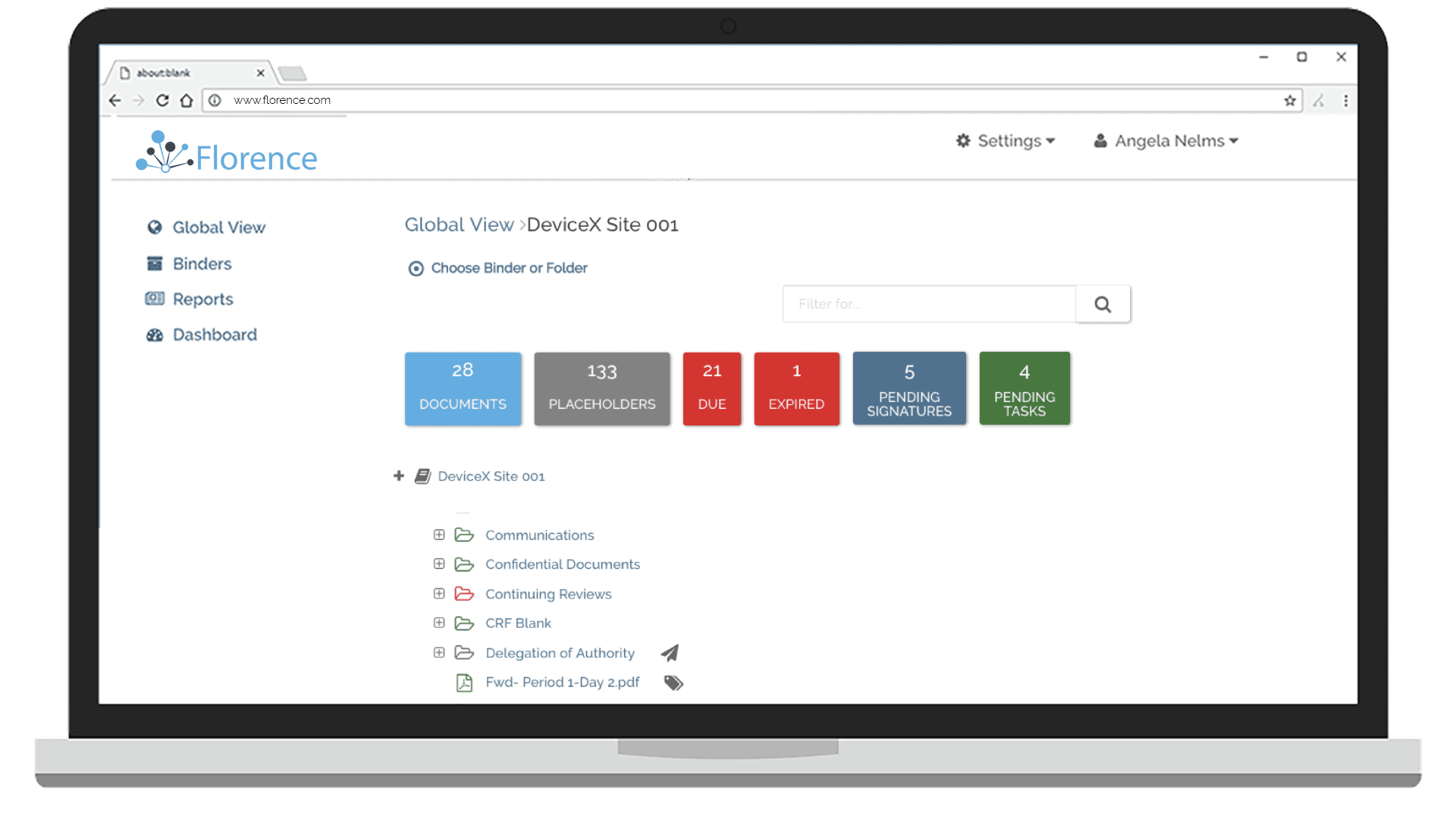
Keep tabs of all of your documents internally and across your study sites. Florence Dashboards provide you a birds-eye view of all document statuses – complete, expiring, expired, and missing – while giving you the ability to drill down to the specific site and assign corrective action tasks to study teams.
Maximize TMF Completeness
70% of your eTMF documents begin their life at the research site. With Florence you have real-time visibility into those documents to take corrective action and maximize TMF completeness.
Ready for a personalized demo?
Click below to let our remote site access experts know and we’ll set-up a quick 15-minute call to answer any questions you have, get an idea of what you’re expecting, and find out if Florence eHub makes sense for your team.