Connect to and Enable Every Site
Accelerate timelines. Increase site capacity. Reduce site burden.
Identify and pre-screen sites for your study with ease. Streamline remote site start-up, monitoring, and source data verification for every location.
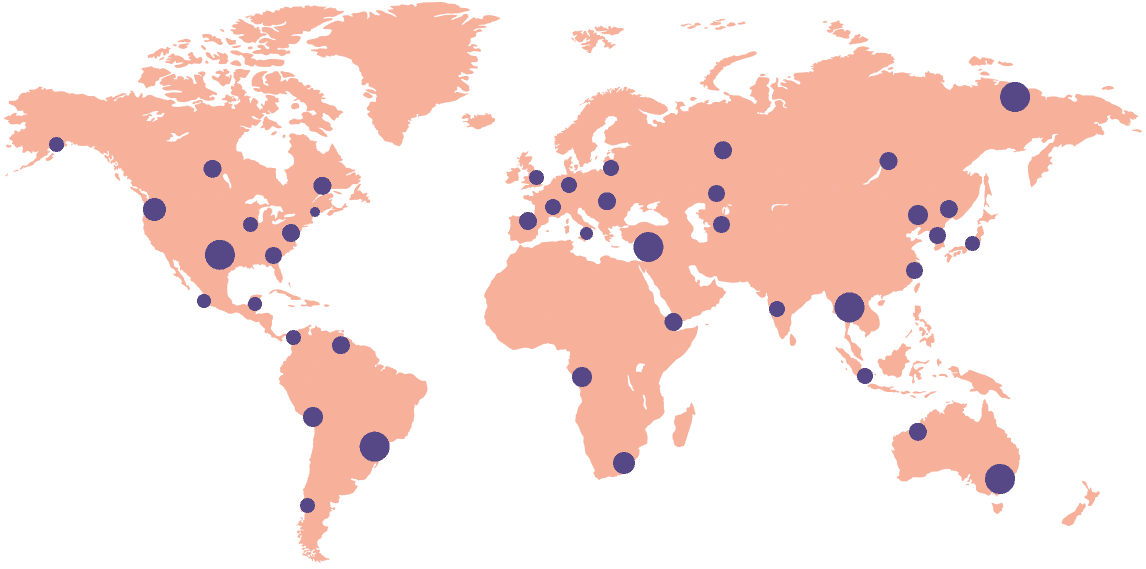
Boost site performance and become the preferred sponsor by utilizing leading eISF and eConsent platforms trusted by 18,000+ sites across 55+ countries. Seamlessly integrate Florence eBinders™, the gold-standard in eISF and Electronic Participant Binder solutions, into your global study.
Why Sponsors Trust Florence’s Site Enablement Platform
The site-trusted solution for connecting workflows and streamlining clinical trial operations on a global scale.
Increase Site Satisfaction
Empower your sites with Florence eBinders via SiteLink, the top-rated platform for clinical trial workflows, acclaimed for its user-friendliness, effortless setup, and exceptional support.
Accelerate Study Start-up
Accelerate your drug development process with our automated workflows. Cut start-up times by 40% and reduce study timelines, all while streamlining your workflow.
Decrease Site Workloads
By providing your sites with eBinders, you equip them with a workflow engine that streamlines redundant workflows and consolidates divergent procedures, leading to a reduction of site workloads by as much as 40%.
Expand Patient Access
Embrace the power of digital connectivity and work with any site, anywhere. By deploying remote digital workflows, you can effortlessly expand your network and collaborate with geographically dispersed sites, enabling you to conduct research with greater ease and efficiency.
Maximize CRA Efficiency
Empower your CRAs with remote access capabilities. SiteLink allows for remote monitoring of over 60 sites per week, reducing staffing bottlenecks and travel costs while increasing impact on study sites.
Improve TMF Quality
Integrate SiteLink with your eTMF for seamless document exchange with sites. Boost your eTMF pass rate from 65% to 98.7% like one of our satisfied customers.
“Florence’s platform is helping Pfizer to respond to the changing environment … and further progress research with the capability to perform remote monitoring where approved by regulatory authorities and ethics committees.”
Rob Goodwin
VP and Head of Operations in Global Product Development
Pfizer
The Site Enablement Platform
Introducing the Site Enablement Platform for Clinical Trial Sponsors. Our name is almost as long as your trials, and its our job to change that. Florence is designed for what sites need in order to deliver what you need.
SITE START-UPReduce Bottlenecks in Site Start-upActivate sites remotely and equip sites with digital workflows that integrate with their existing workflows. Deploy the #1 rated eISF platform to sites to enable study start-up document routing and real-time collaboration in just a few clicks. Monitor performance and activity across all sites in a single location. |
SITE CONDUCTSimplify FPI to LPODigitize and integrate every document workflow from first-patient-in to last-patient-out, reducing site burden by up to 40%. Enable sites with the best-in-class eISF (eBinders) and eConsent platform to streamline their operations while activating always-on remote monitoring capabilities, advanced site performance insights, and real-time document exchange with the eTMF. |
SITE CLOSE-OUTSigned, Sealed, SubmittedClose out your studies on time with improved TMF submission quality, compliance, and completeness. Integrate site documents with the eTMF through automated quality control workflows, using seamless methods like API integrations, email, and drag-and-drop. Equip sites with long-term archiving included. |
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
18k+
Sites activated on the platform
55+
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Make Sites Happy
(Your CRAs Will Thank You Too)
Enable Sites, Accelerate Trials

E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.
Trusted by Sponsors,
Loved by Sites



















