STUDY PLANNING
Plan for Success
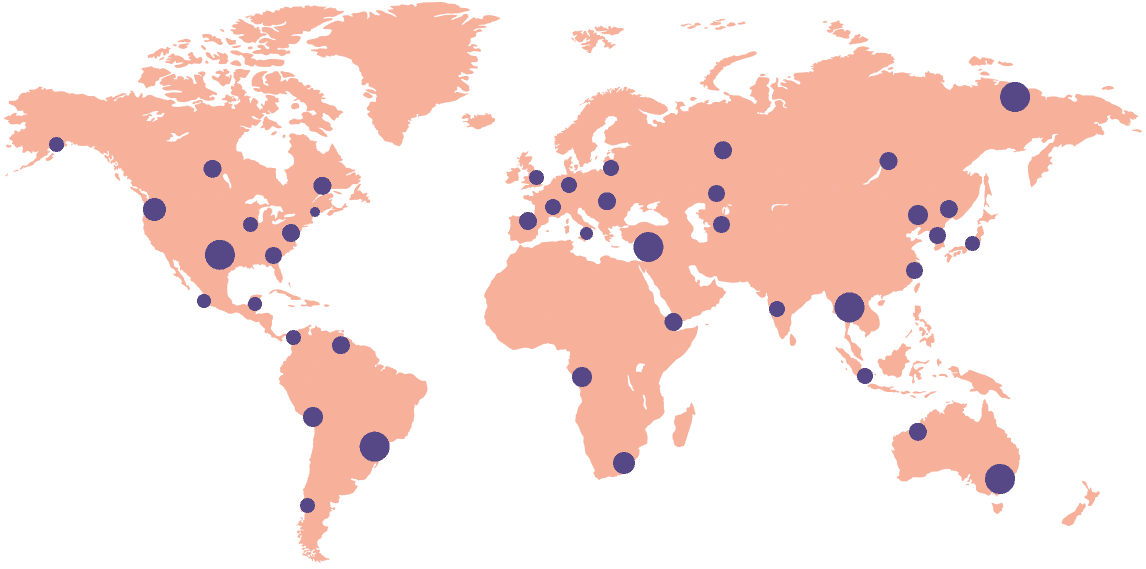
Search for sites to participate in your study in our Site Navigator Network, linking 12,000 sites in 45 countries.
Then, pre-screen sites based on pre-filled and up-to-date feasibility profiles. Now you can choose the sites that best meet your needs, and begin your study with the greatest chance of success.
How Florence Streamlines Study Planning
Start your study process on the workflow platform that digitizes the site-sponsor connection across 18,000+ sites in 55+ countries.
Identify New Sites to Partner With
Explore the network of 12,000+ sites across 45 countries that are connected to the Florence platform to identify potential locations to launch your study.
Pre-Screen Sites
Review pre-filled site feasibility questionnaires to ensure the basics of the site meets your protocol criteria before launching a time consuming process.
Reduce Feasibility Friction on Sites
Connect with sites on the platform they trust, access pre-filled feasibility questionnaires, and reduce workflow friction at the site related to document workflows.
Accelerate Site Start-up
Get a jump-start on the start-up process by connecting with sites for feasibility within the Florence end-to-end platform. Once a site is confirmed, sending start-up documents is simple in SiteLink.
Expand Patient Access
Identify frontier sites, those new to research, that have access to patients to meet your DE&I initiatives and match your complex protocol requirements.
Reduce Competition for Sites
Established sites are becoming more competitive to get studies placed. Supplement your site mix with access to thousands of research sites across the globe.
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
18k+
Sites activated on the platform
55+
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Enable Sites, Accelerate Trials

E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.
Trusted by Sponsors,
Loved by Sites

Make Sites Happy
(Your CRAs Will Thank You Too)















