Streamline your clinical operations with a native SIP connection to the largest eISF network in research, Florence, now hosting over 8,500 study site eISFs in 30+ countries.
Together, Florence and SIP deliver a site-first integrated digital experience through their strategic relationship and exclusive native integration.
The SIP eISF Connect module automates the flow of documents between the sponsor eTMF systems and Florence eBinders, the leading electronic Investigator Site File System (eISF), while enabling remote site access for monitoring and SDR/V.
Learn how you can take advantage of this native integration today.
How does the SIP to Florence integration work?

Integrate your eTMF Structure with Site eISFs
Eliminate the need for mailing, emailing, and faxing study documents, and legacy site upload portals, by directly integrating your eTMF structure with your sites’ eISF.
Enable Real-Time Site Oversight
Comply with ICH GCP E6(R2) requirements for site oversight


Forecast Site Performance
Know where all of your sites stand with their startup and study progress in real-time.
Keep Sites on Track
Easily give CRAs the ability to assign tasks and specify deadlines for sites.

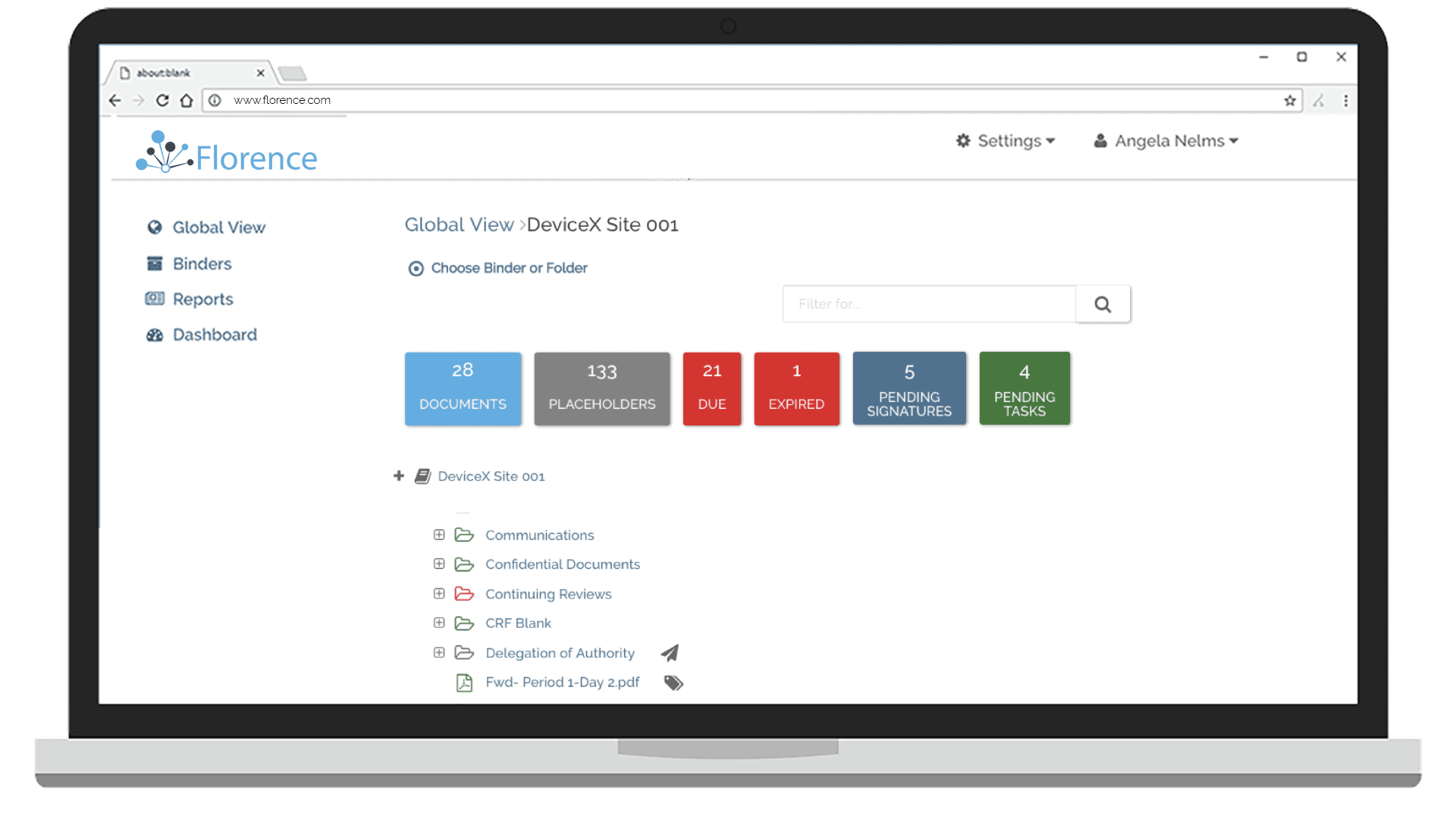
Monitor Site Progress and Source
Gain real-time insights into individual site, and study-wide, progress and source documents.
Quality Control Site Documents
Ensure document quality before syncing back with your eTMF structure.








