
Clinical trials will grow 5.8% from 2022-2030. To meet this growing demand, research sites, sponsors, and CROs will deploy purpose-built technology solutions.
Research sites are moving away from sponsor-first digital platforms (software designed for sponsors and CROs and forced on sites). Instead, they’re investing in site-first infrastructure across their research ecosystem.
Site-first solutions focus on research site teams, workflows, and processes. Emphasizing sites when building eClinical solutions streamlines workflows, lowers costs, reduces compliance errors, and enables scale – ultimately accelerating the timeline of new medical advancements reaching patients.

Sponsors and CROs also benefit from the site-first eClinical design approach. Sponsors’ systems can now link seamlessly with platforms at the site – resulting in sites working with a platform they are familiar with and enjoy using.
While numerous categories of eClinical solutions exist, this guide focuses on the solutions used in clinical trial site document and data management.
4 Key eClinical Benefits for Clinical Trial Sites
The benefits of introducing eClinical solutions at the research site go beyond merely moving out of paper.
Integrated and interconnected platforms introduce a new level of efficiency, improved regulatory compliance, and connectivity. Sites are placed at the center of the ecosystem while integrating directly back to sponsors and CROs for real-time document and data exchange and monitoring.
Integrated and interconnected eClinical platforms at the research site result in greater efficiency, improved compliance, and real-time connectivity.

The Electronic Investigator Site File (eISF) and other site solutions store all documentation generated before, during, and after the study at the research site. All documentation must be complete, legible, and easily accessible at all times for monitoring and audits.
In addition to an eISF, many sites also rely on eSource (electronic Source) and eConsent (electronic informed consent) to make their workflows more efficient.
Trends and Components of the eISF and Other Site Solutions
Historically, if a research site maintained an “eISF,” it was as part of a sponsor or CRO-deployed electronic portal. This approach, while beneficial for the sponsor or CRO, adds little value to the research site – and could even add to sites’ workloads, as sites must interact with dozens of different eISF platforms across sponsors and CROs.
The idea of eISF platforms designed specifically for sites became popular in 2019 and exploded during the COVID-19 pandemic. Site solutions like eISF, eConsent, and eSource continue to grow in popularity.
6 Key Benefits of a Site-first Platform
Eliminating paper and disconnected systems aren’t the only reason to invest in a site-first electronic Investigator Site File.
When deployed correctly and integrated with existing systems, the eISF has the potential to transform the way research sites operate and their relationship with sponsors and CROs.

An Electronic Regulatory Document Management System (eRegulatory) is an essential component of a fully functioning digital ecosystem at the clinical trial site. eRegulatory manages all of the regulatory-related documents created and maintained before, during, and after the study.
Primary Features of eRegulatory

eSource is the platform that captures the initial source documentation of subject data in the clinical trial. Instead of recording source data manually and then later transferring data to an online system, eSource captures data electronically from the start.
Methods of eSource Capture
EHR/EMR (Electronic Medical Records/Electronic Health Records): Integrate directly with the EMR/EHR to seamlessly capture the source documentation from the electronic health record.
Direct Data Capture: Direct data entry by site staff into a mobile application or EDC system.
Devices and Applications: Collection of data from non-site staff using mobile devices, wearables, and sensors.
Non-CRFs: The collection and transfer of data from sponsor sources (ie: laboratories) or external vendors.
To learn more about types of eSource, check out our comprehensive article.

Primary Features of eSource

An eConsent system offers a digital platform that patients can use to read, sign, and submit their informed consent documents.
The FDA allows the use of eConsent, as long as that eConsent is compliant with clinical trial-specific regulations. Below is a quick list of features you should look for in your eConsent.
And if you’d like to learn more about what to look for in eConsent, check out our eConsent evaluation checklist.
Primary Features of eConsent

The CTMS provides a single, centralized system to manage operational and administrative activities in a clinical trial.
A CTMS is used by sponsors, CROs, and research sites to manage critical operational tasks across project management, facility/staff capacity planning, financials, subject tracking and scheduling, and protocol adherence.
Although Florence doesn’t have a CTMS product, we perform integrations with many CTMS vendors who have an open API. Learn more about integrations and an open API here.
CTMS Trends
Clinical Trial Management Systems are among the first site-based eClinical solutions, and roughly 70% of sites use them as of 2022.
While Florence does not have a direct CTMS solution, we believe in the importance of eISF-CTMS integration. You can learn more about the types of integrations we support here.
Primary Capabilities of a CTMS per User Group

A sponsor or CRO might deploy an Electronic Data Capture (EDC) system for the collection of clinical trial data at the research site in an electronic format. Data is typically recorded first at the site on paper documents, before being transcribed into an eISF and saved in an Electronic Case Report Form (eCRF). EDC removes the paper step.
This technology is still new and not commonly used. But it can be seen as a very specific category of eSource.
EDC Trends
As the eSource platform continues to evolve and become a staple at the research site, we predict sponsors and CROs will begin integrating their data platforms directly with site eSource platforms, eliminating the need for duplicate manual transcribing of data.
79% of sponsors and CROs and 70% of sites indicate that they expect to use eSource by the end of 2022, making this a growing but not universal technology.

Primary Capabilities of an EDC
Demand for site-sponsor links has been increasing, with sponsors and CROs listing remote monitoring as their number-one challenge to solve in 2022 and 2023. In response, software vendors serving the sponsor or CRO market have deployed “sponsor-first” software platforms, such as site portals, at the research site.
These platforms place the sponsor/CRO at the center of the ecosystem and generally add little value to the research site–which is why they have low adoption rates among sites.
We need a new model, one that gives sites eISFs they own and love to use–and then gives sponsors and CROs seamless, always-on links to those eISFs.
Florence is pioneering these links with Florence SiteLink™. SiteLink directly links sponsors and CROs to research sites’ eISFs, so sites, sponsors, and CROs can collaborate and exchange documents, data, and messages instantly.
You can find information about how sites and sponsors can collaborate to make clinical trials more efficient here.

Primary Capabilities of SiteLink™
Florence Solutions Overview
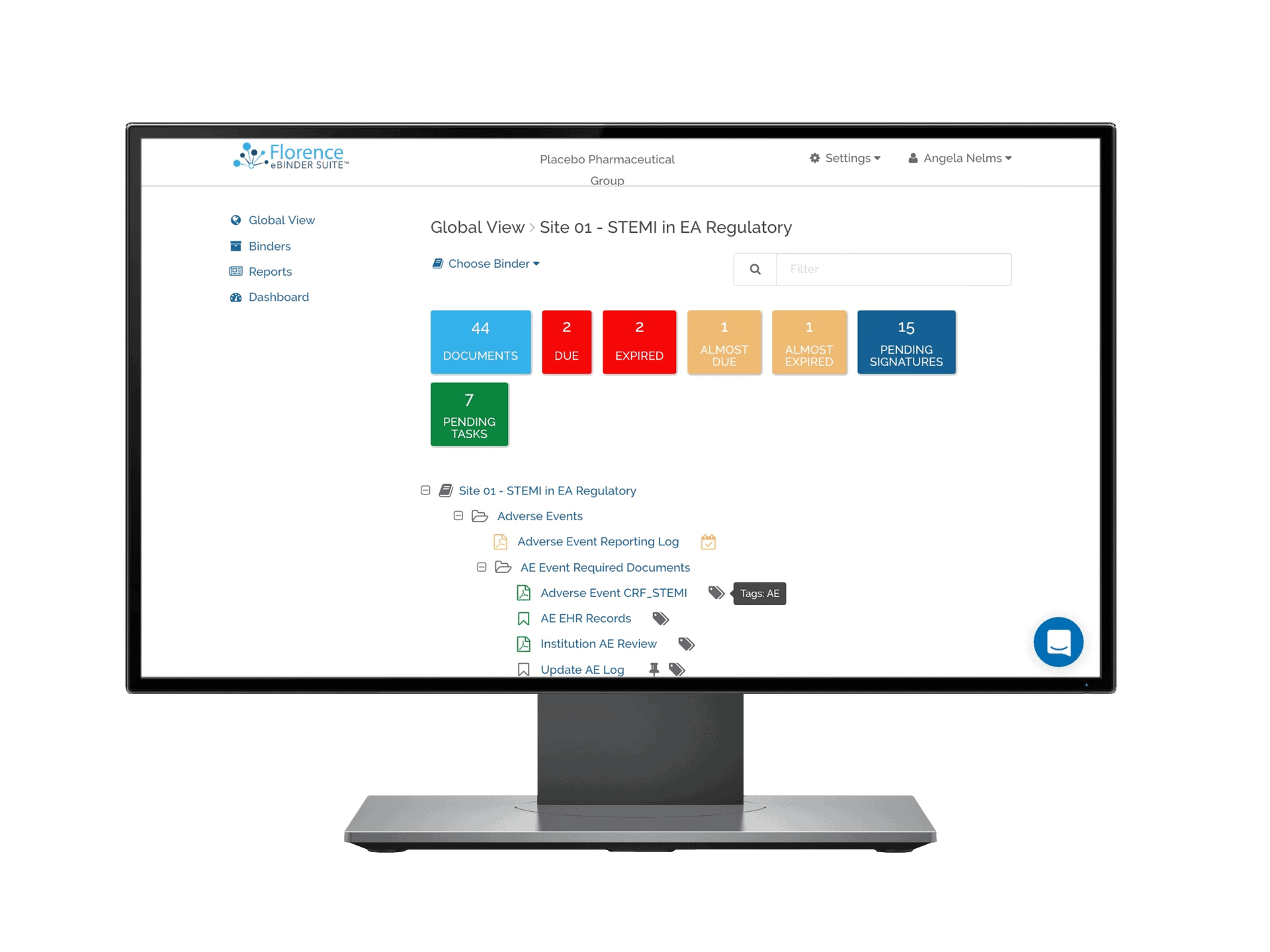
Florence eBinders – eISF
Florence eBinders™ is the eISF of choice for leading Research Sites. In use by over 10,000 Research Teams around the world, Florence eBinders™ features site-first eRegulatory, eSource, and eConsent capabilities.
Florence eTMF
Florence eTMF is the only eTMF on the market with the capability of directly integrating with over 10,000 Research Site eISFs. This feature-rich platform is designed for the sponsor/CRO that needs maximum flexibility and ease-of-use.


Florence SiteLink™
Florence SiteLink™ is the only connectivity platform that connects the sponsor/CRO eTMF directly to the Research Site eISF. Sponsors/CROs gain advanced site operational analytics, remote monitoring capabilities, and document/data exchange features.































