Selecting an eTMF Vendor that Empowers Site Collaboration
Not all sponsors and CROs that select and implement an Electronic Trial Master File (eTMF) solve the bottlenecks and inefficiencies that the system was selected to target.
Why?
Often, during the vendor selection process, organizations undervalue the impact of vendor support teams, overlook critical implementation processes, and don’t fully understand the correlation between software capabilities and the bottlenecks they address.
The biggest challenge that ultimately affects how well an eTMF system performs is its ability to drive site collaboration and integrate with the tools sites are already using.
With over 10,000 research sites connected to the Florence Network, we understand the delicate relationship between the sponsor/CRO and the site and how a digitally integrated eTMF and eISF can significantly impact the overall success of a Clinical Trial.
To help guide sponsors and CROs through a smoother and more fruitful eTMF process, we created this article to address common friction points, such as:
- Selecting an eTMF Vendor
- Implementing an eTMF
- Site Collaboration
Download the Complete Guide to the eTMF to get an in-depth overview of how a modern eTMF solution can impact your organization, the compliance and regulatory system requirements, and the steps you can take to prepare for adoption.
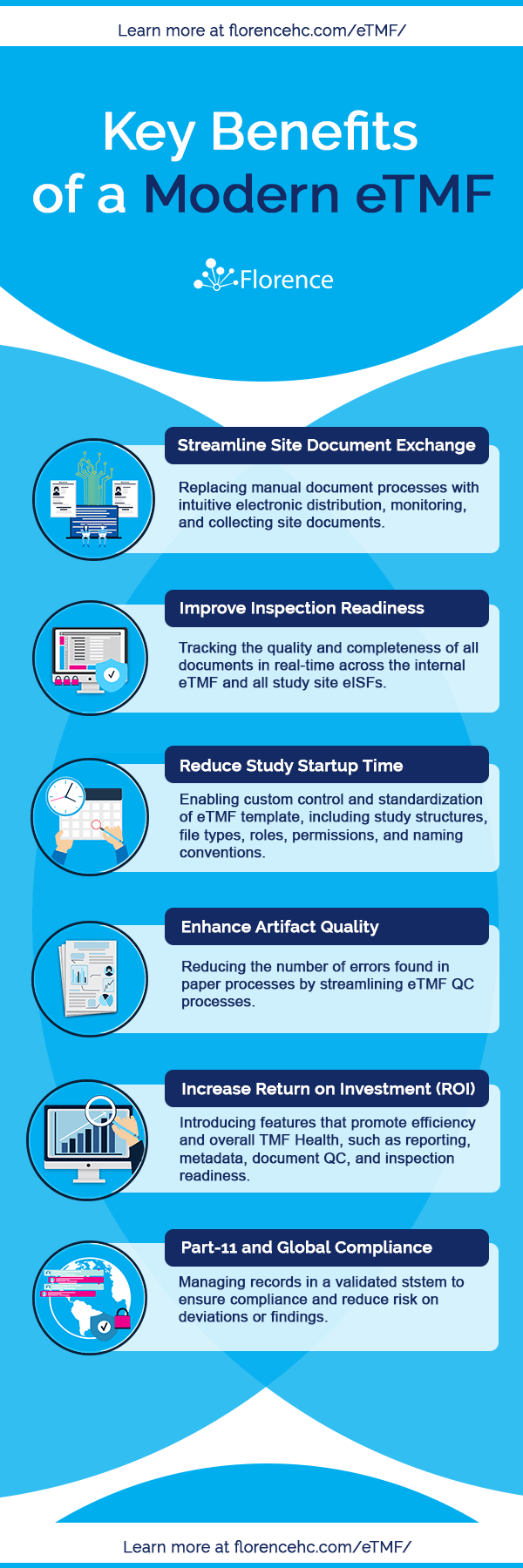
Selecting an eTMF Solution
As you begin to evaluate and plan for an eTMF, it is essential to have a strong understanding of the capabilities you require to address the unique bottlenecks and inefficiencies at your organization.
Every platform is unique. However, there are essential assets you should look for that will enable you to realize the benefits of a modern, active solution. These are the core areas to focus on when evaluating a solution.
Implementing the Modern eTMF to Drive User Adoption
It is typical for you to introduce the selected eTMF to all your clinical trial stakeholders for the first time during the implementation process. A disorganized process with little vendor support will negatively impact how broadly the new system is adopted, accepted, and utilized.
Widespread user adoption will largely determine how effective the system is at improving process efficiency, reducing redundant tasks, minimizing costly delays, and maximizing financial return on investment.
The two lists below outline the most critical do’s and don’ts of implementation based on field-tested practices of implementing technology for clinical trials at hundreds of sites.
What to do:
- Configure your folder and document organization according to your needs.
- Look for ways to standardize across multiple studies and sites.
- Inquire about best practice recommendations and templates.
- Consider a crawl-walk-run approach to assist in change management.
What not to do:
- Come out of the gate too hot; you run the risk of overwhelming your team which can hinder your success rate.
- Not expect your vendor to be committed to your success. You shouldn’t have to do it alone or rely on consultants.
We suggest evaluating each vendor during the vendor selection process to determine if their implementation support is sufficient to guide your team to success.
When planning the implementation process to transition your team from traditional paper and fragmented technology workflows to a fully electronic and purpose-built eTMF platform, there is a collection of best practices that create a conducive environment to ensure user adoption of the new technology.