Welcome to Florence!
Your sponsor or CRO has chosen Florence eBinders™ to support your study, helping you streamline clinical trial management with the leading electronic Investigator Site File (eISF) solution.
Explore the resources below to discover how eBinders simplifies workflows, enhances collaboration, and ensures compliance—all while keeping your study on track.
Get Started
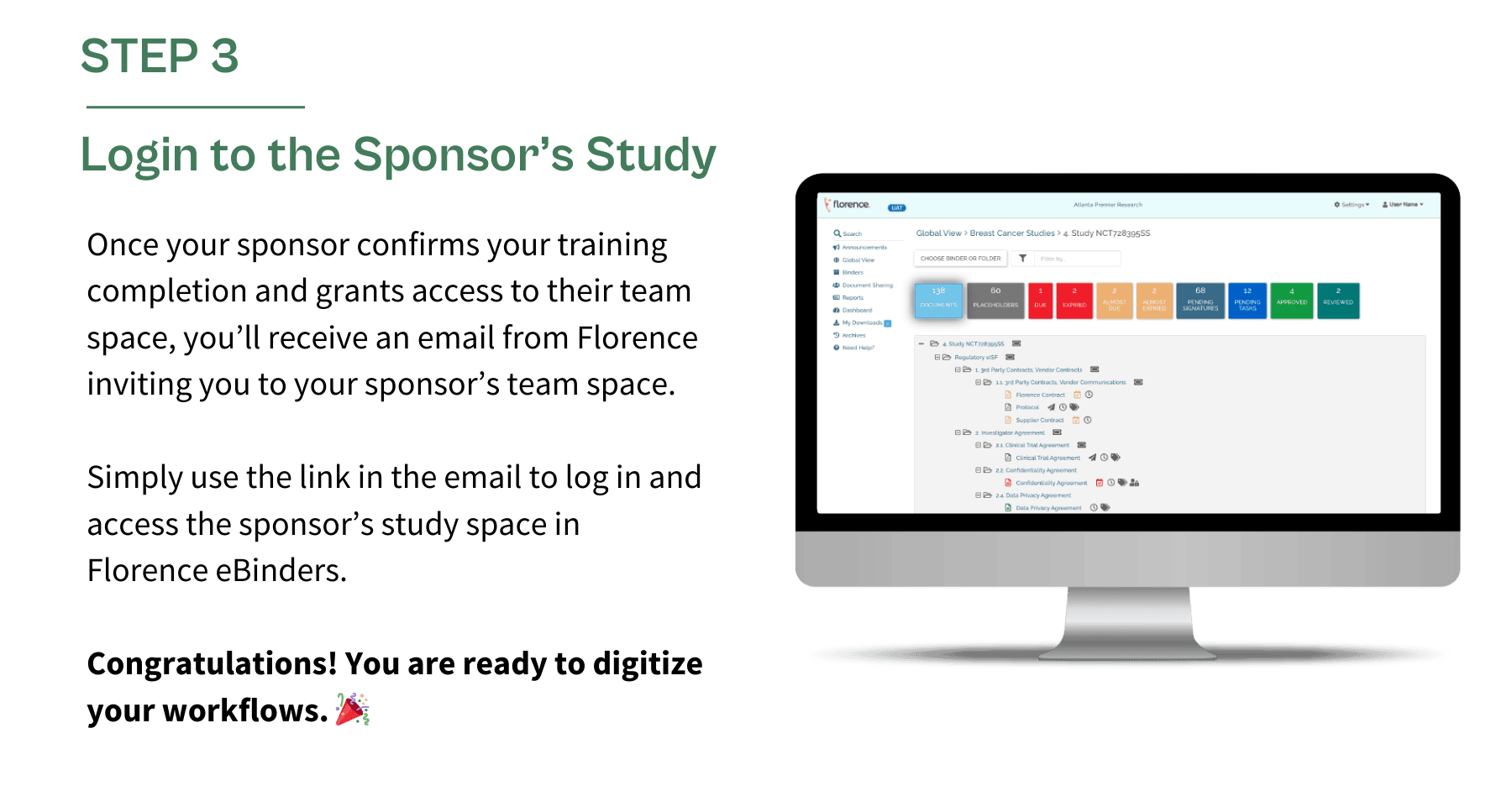
Once your remote collaboration space is ready, follow the steps below to get started.
Resources
Dive into additional learning materials, templates and guides on Florence features and workflows.
*Please note: Some resources require login. If you’re already using eBinders, check your welcome email for a link to your study-specific resource page, which may include additional tools.
Compliance
Florence Support
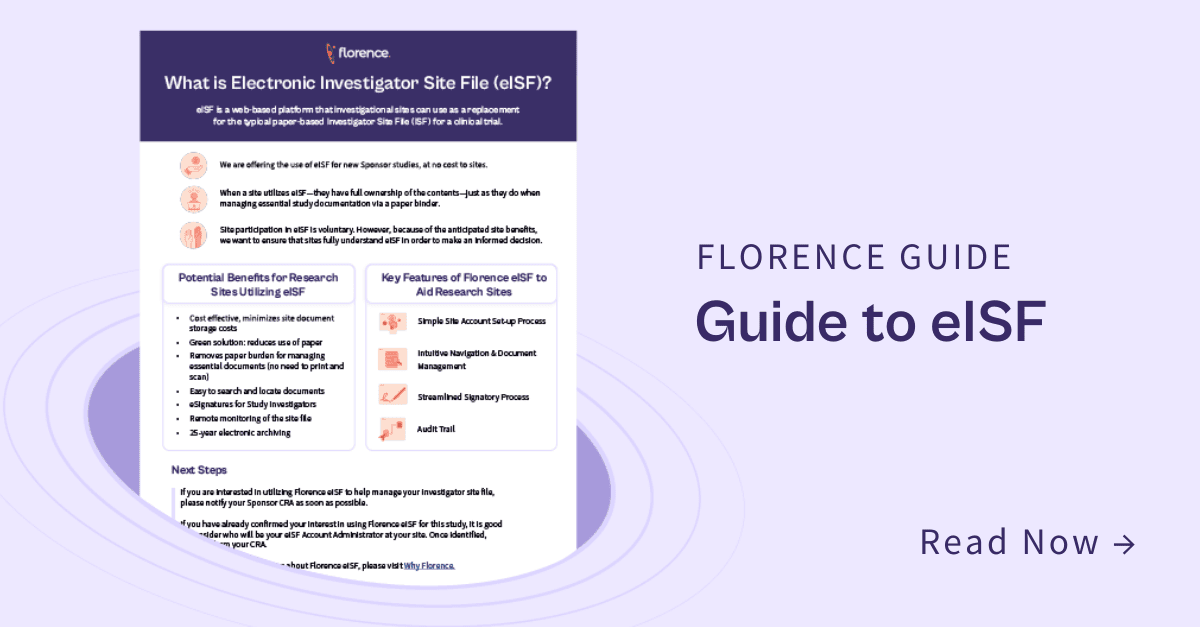
Benefits of Using Florence eBinders
A compliant electronic version of your Investigator Site File
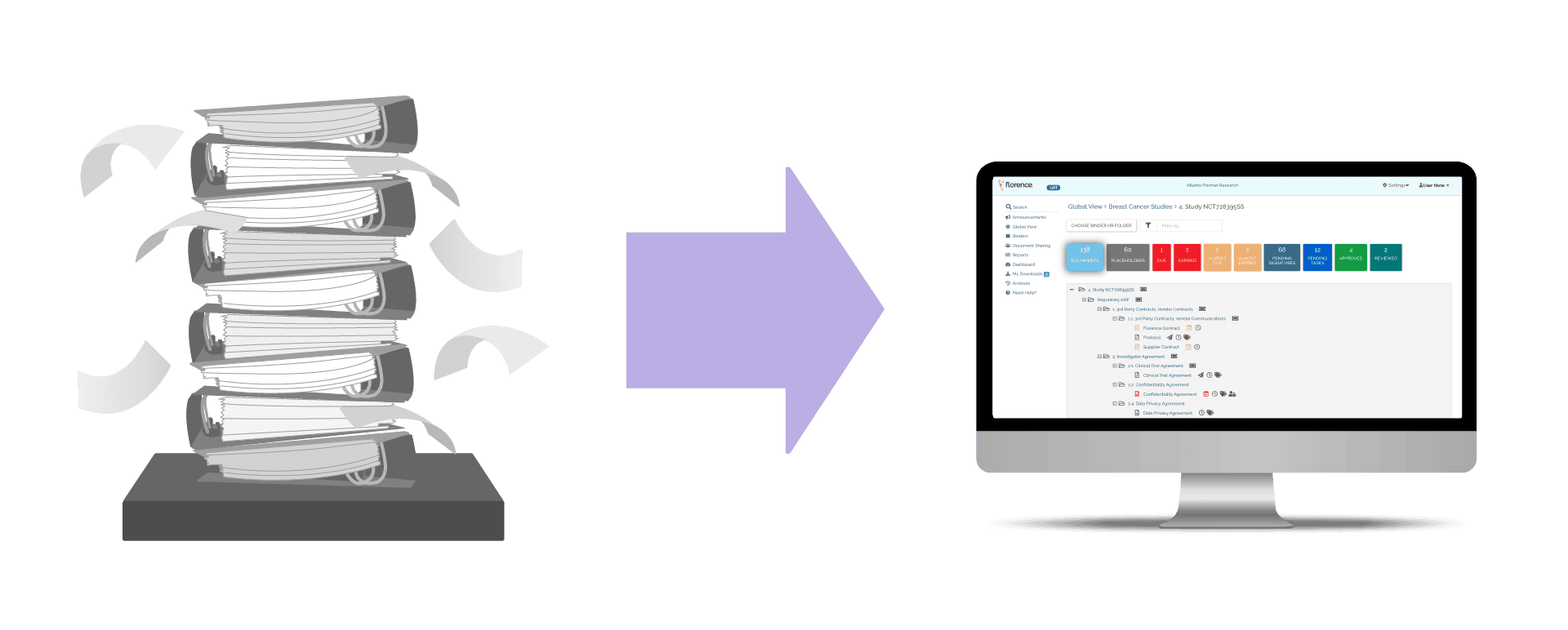
Transition from paper-based ISFs to a site-controlled, web-based system for effortless creation, management, and sharing of essential regulatory documents.
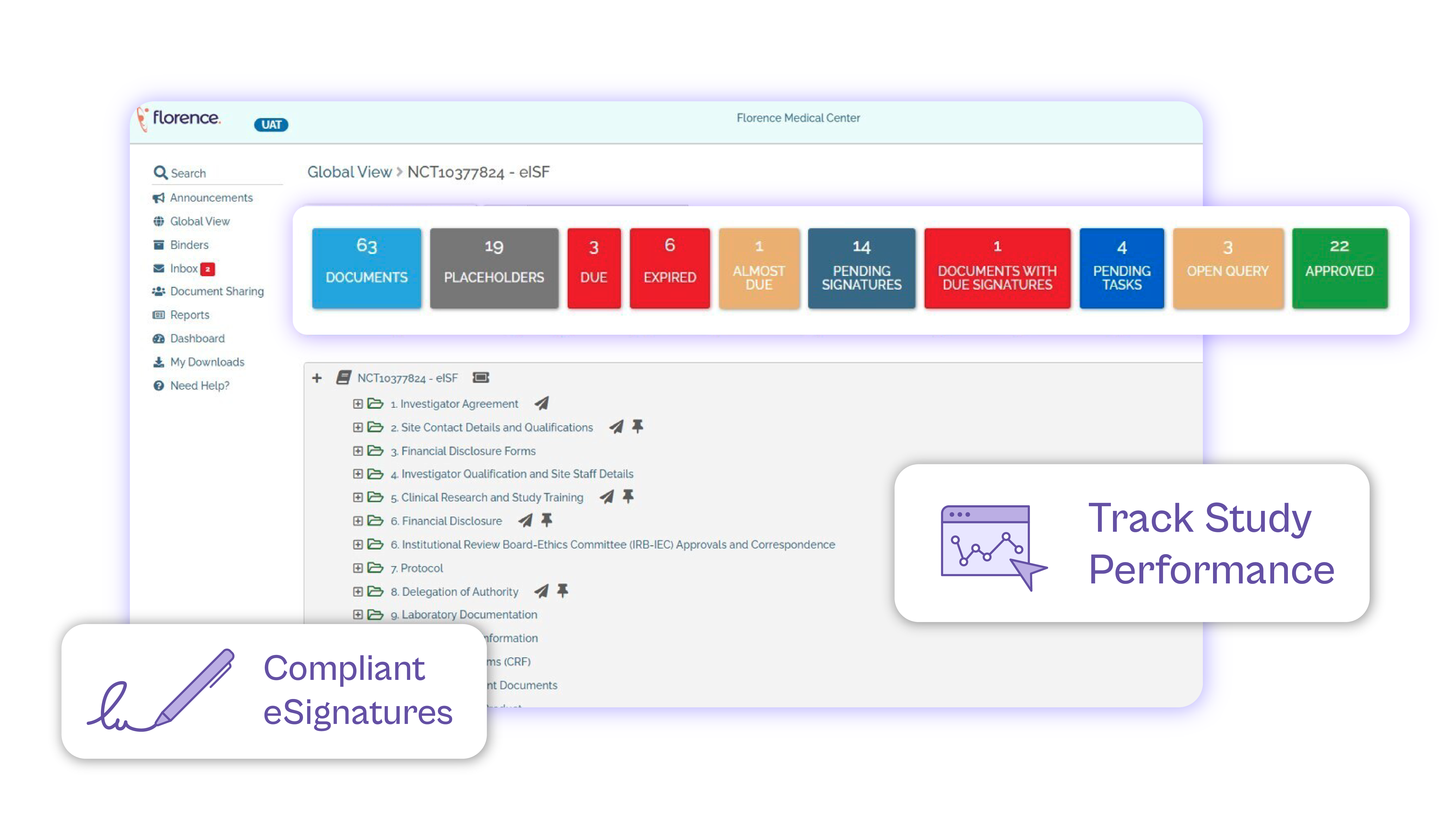
Empower monitors with remote access to review eISF content before on-site visits, facilitating smoother collaboration and preparation.
Experience the efficiency and compliance benefits of automated workflows, utilizing audit trails as a reliable source of truth and enabling PIs to sign documents conveniently from any location.
With Site Love
No Cost for Sites
We’re providing eBinders at no cost, emphasizing our commitment to seamless document collaboration and remote monitoring.
Global Trust
Adopted by over 20,000 site teams across 90+ countries as the preferred ISF with a best-in-class customer satisfaction rate of 97.6%.

Ease & Efficiency
Designed to be the most user-friendly eISF, automated workflows and intuitive search optimize document management, eliminating paper binder complexities.

Dedicated to Your Success
Rated #1 on G2 by sites, compliant with global regulations, and backed by 24/7 support and online resources.
In clinical research, compliance is crucial. We’ve got you covered.