The Overall Organizational Impact of a Modern eTMF
Implementing an eTMF is critical for Sponsors and CROs that want to reduce burden, increase study capacity, and enable more trials. However, many organizations are not equipped with a modern solution to realize the full benefit and future proof operations.
With over 10,000 research sites connected to the Florence Network, we have an intimate understanding of the research site and the site’s Electronic Investigator Site File (eISF), where over 80% of a TMF’s documents originate.
Through this uniquely qualified lens, we have compiled this article and an accompanying downloadable guide to evaluating an eTMF covering:
- Impact of a Modern Active eTMF
- Benefits and ROI
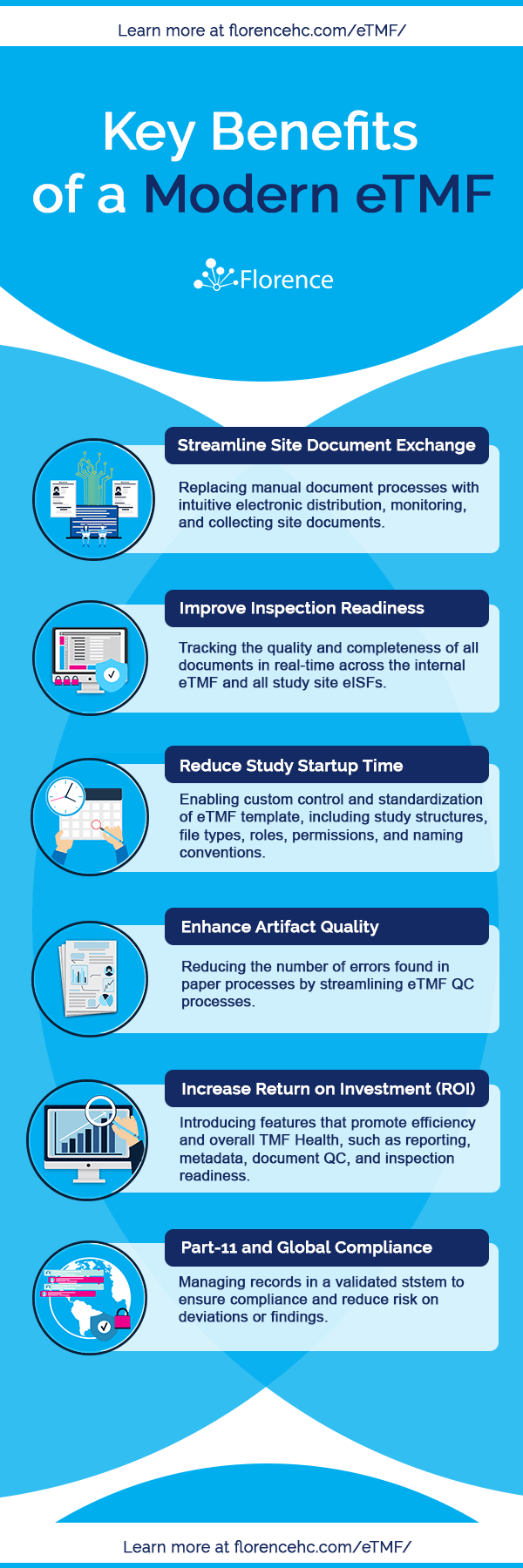
The Impact of a Modern eTMF
Eliminating paper processes isn’t the reason to pursue an eTMF solution. Instead, the benefits of a modern eTMF solution enable organizations to manage the increase in study complexity that has been seen over the past decade and remove the research site silo from operations.
The most prominent benefits experienced by adopting an eTMF solution are:
Download the Complete Guide to the eTMF to get an in-depth overview of how a modern eTMF solution can impact your organization and understand the steps you can take to prepare for adoption.
The Key Benefits and ROI of a Modern eTMF
Implementing an eTMF solution is an investment. Realizing a return on that investment requires ensuring that the solution you choose addresses basic eTMF workflow needs in a modern, ever-evolving landscape.
A Modern eTMF will:
- Eliminate paper and create a more sustainable organization with ever-changing regulations around archival.
- Virtualize eTMF documents to reduce study management costs considerably.
- Streamline processes that drive increased study capacity and the ability to get drugs to market faster.
- Enable document exchange coupled with real-time collaboration to create fluid workflows and save costly time.
- Provide direct hard-cost savings with a reduction in physical documents to manage, therefore freeing these resources to be shifted to other organizational needs.
- Give CROs a clear advantage over the competition as it enables the ability to manage large multisite trials without adding headcount.
To better understand the overall impact of an eTMF system of day to day processes, we outlined the key benefits to a modern eTMF below.
The Decision is Yours
Deciding to implement an eTMF system at your organization is no small task. The upfront work, overall impact, and projected return on investment must all be analyzed before deciding to move forward with the vendor selection process.
Using the information you have gathered here, it is now time to begin applying these data-driven insights to your organization.
Will the key benefits and overall impact make a difference at your organization? What are the existing bottlenecks and costly processes that keep your team from productively conducting clinical trials? The decision is yours.
To continue learning about the eTMF through vendor selection, compliance and regulatory, implementation, and site collaboration – please download the Complete Guide to eTMF here.