How to Overcome Remote Monitoring Challenges in Clinical Research
The global pandemic has shifted the clinical research ecosystem towards the rapid adoption of remote site monitoring. This shift has brought challenges for both monitors and sites trying to navigate new systems, processes, and regulations.
Although remote monitoring enables easy site access, remote patient visits, and decentralized trials, monitors and sites face numerous challenges when selecting, implementing, and developing a successful strategy.
Many leading sites, sponsors, and CROs have overcome these challenges. Andrea Bastek, Director of Innovation at Florence, is familiar with the fear many organizations face when considering the implementation of remote monitoring.
We sat down with Andrea to examine what makes the process hard and help us uncover solutions to address stakeholders’ concerns across clinical research.
Why has COVID-19 accelerated the adoption of remote monitoring in clinical research?
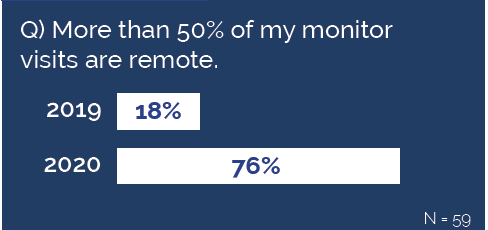
Remote monitoring adoption has increased, mostly out of necessity. The Florence 2021 State of the Industry survey showed that 76% of sites and sponsors reported >50% of monitoring visits are now remote, compared to only 18% last year.
In the early days of COVID, creativity, and collaboration often sourced quick low-technology solutions like:
- Emailing documents
- Screen sharing with site staff to view EMR data
- Scanning and uploading source documents
As site restrictions tightened and limited the ability for monitors and even site staff to be on-premise, organizations began to investigate and adopt more permanent technology solutions like remote monitoring and remote site access platforms.
What obstacles do sponsors and CROs face when selecting a remote monitoring technology?
From my experience, a sticking point for sponsors and CROs tends to be their internal monitoring plan requirements. For example, if a monitoring plan requires Source Data Review (SDR/V), the sponsor needs EMR access. If their selected platform does not support SDR/V then this presents a big challenge. Our data shows that direct EMR access is still only available around 30-40% of the time.
Changing monitoring plans takes time and can be tough to adjust once the FDA has accepted them.
Another hurdle is the variability from site to site. For instance, some methods may be available and approved at one participating site but not another. In our experience, sites that had never previously conducted remote work weren’t equipped to implement remote solutions quickly.
A final consideration is that all remote monitoring technology is not created equal. Some solutions do not always satisfy the need for SDR/V, and, in some cases, the technology is not compliant with all pertinent regulations.
To navigate these obstacles, the technology evaluation process must be thorough and demonstrate the selected platform’s ability to support the sites’ existing technology infrastructure. In other words, interoperability is critical to ensure site adoption.
How has remote monitoring technology changed the clinical research landscape?
Using technology platforms to interface between sponsors, CROs and sites created efficient communication and data transfer that didn’t exist before.
Sites that were already using electronic processes were better prepared to adopt new remote monitoring technologies or align with new monitoring workflows. Some sites could even facilitate remote monitoring by extending their eISF platform access to sponsors and CROs.
Technology has the power to streamline workflows, improve efficiency, enable real-time oversight, and reduce administrative burden – but only if the systems are robust and the integrations are thoughtful.
What hurdles do sponsors and CROs face when driving a remote monitoring implementation strategy?
To drive study and data continuity, first sponsors and CROs may have to adjust their internal remote monitoring methodologies, risk assessments, analytics, and monitoring plans. After the administrative tasks are complete, they can begin developing a plan to implement the new technology. Most vendors should work hand-in-hand with the organization during this process.
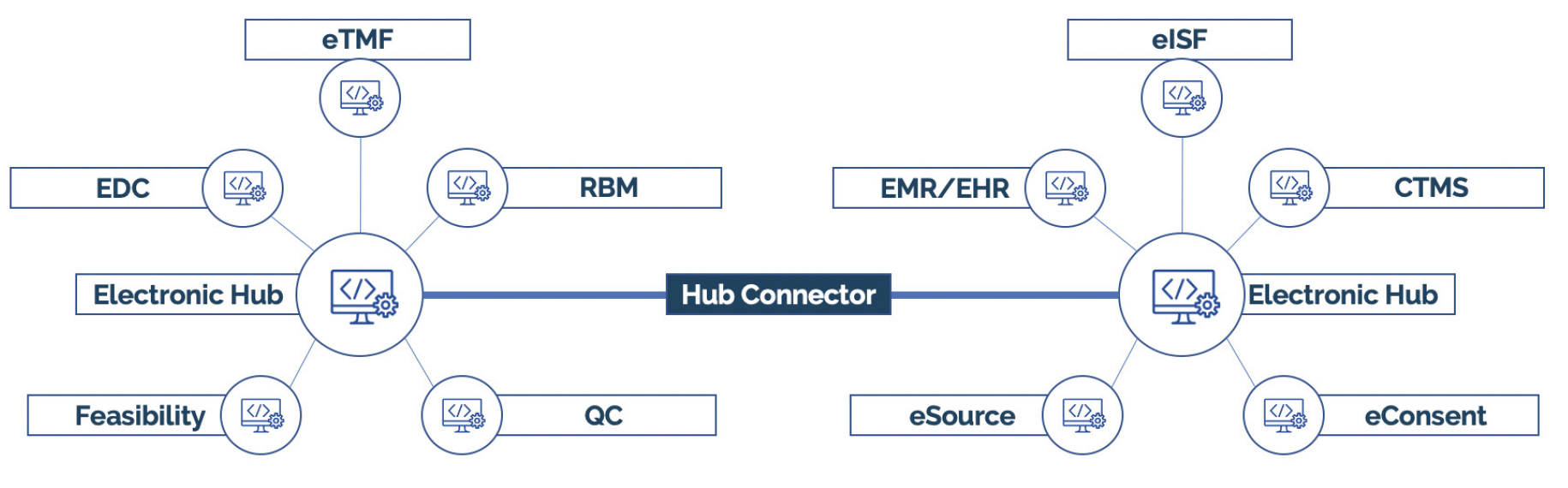
 One snag in this process can be integrating new remote monitoring technology with existing software platforms. Every site is unique, and many already use eRegulatory, eISF, and CTMS solutions, not to mention the variety of EMR systems that exist.
One snag in this process can be integrating new remote monitoring technology with existing software platforms. Every site is unique, and many already use eRegulatory, eISF, and CTMS solutions, not to mention the variety of EMR systems that exist.
Without robust integrations and integrated workflows, implementing new technology could cause duplicate work. To overcome this challenge, choose a vendor who is committed to integrations and interoperability is key.
Another concern is the risk of low user adoption. New tools require training and practice to become efficient. A vendor with a proven implementation process, role-based training plans, and a clear support strategy reduce this risk.
A final challenge is verifying vendor risk management and Quality Assurance (QA), which is often a huge concern for sites. Many sites aren’t familiar with using or evaluating technology vendors – so it can be overwhelming when a sponsor or CRO comes knocking with new technology.
However, you can resolve these fears by identifying a vendor that not only abides by necessary regulations but helps enforce them through their technology and workflows and offers resources and an open dialogue to address questions and concerns.
Remote monitoring can include the monitoring of sites and patients, as well as hybrid or virtual decentralized trials. How can we ensure ‘patient-centricity’ in these trials? Is this an increased focus of technology vendors today?
We can look at this question across the entire stakeholder ecosystem:
Participants: Moving to hybrid or virtual trials often adds convenience for the participant. Eliminating geographic boundaries and language barriers can give participants access to a much broader range of studies. If the technology is user-friendly and engaging, there may be an additional benefit of increased compliance and reduced participant drop-out. To avoid alienating potential participants, technology must be thoughtfully designed to accommodate all users and bypass creating biases in clinical trial recruitment. For example, there are significant socio-economic implications to using complex technology, or technology that requires smart-phones or home internet. Some solutions with direct participant usage are wearables, electronic pill counting, eConsent, and eCOA/ePRO.
Sites: Some key concerns sites have for remote solutions are reduced face-time with patients, potential loss of patient care opportunities, reduced ownership of trial data used for clinical decision making, and maintaining PI oversight in a remote setting. Sponsors and vendors should consider these concerns and find solutions that address them and maintain patient-site engagement. However, the potential benefits of expanded patient catchment areas, more diverse participant groups, and ultimately advancing cures targeted at larger populations may outweigh the concerns. We’ve also seen research sites leverage these advanced technology capabilities to open additional rural or off-site satellite locations with smaller headcounts while maintaining some in-person care and oversight.
Sponsors: Historically, studies with more remote patient visits were more challenging to receive FDA approval. With the increased adoption and acceptance of remote solutions in healthcare, we expect a positive shift in how the FDA views these modernized trials. You can learn more about the general benefits of remote site monitoring for sponsors and CROs in this article.
Vendors: Yes, many technology vendors are focused on participant-centricity. Perhaps more than ever, vendors consider the impact their solutions and future innovations have on participants and stakeholder dynamics. A good vendor manages its product roadmap to ensure patients and participants are at the forefront via thoughtful UX design, accessibility, integration ability, and ease-of-use.
Moving Forward
Critical to any successful remote monitoring strategy is selecting a platform that is compliant, integration-friendly, and easy to use. Your vendor should be a partner in the implementation process and throughout the life of your relationship together by offering ongoing support, a robust product roadmap, and open channels for feedback.
The obstacles of remote monitoring span across the stakeholder groups in clinical research. Any technology advancement is destined to create new challenges among the complex systems and processes of clinical research, but once overcome, the benefit can far outweigh the fear.
If you are searching for your own platform, this Remote Site Access Evaluation Checklist has over 10 pages of thoughtful capabilities you should consider when evaluating vendors.

