Decentralized Clinical Trials | What the Future Holds
The concept of decentralized clinical trials is not new. But the COVID crisis forced research operations leaders to accelerate their plans for remote and hybrid trials.
Decentralized clinical trials have now become one of the most important innovations in clinical research. But the meaning of the term “decentralized clinical trials” is still not universally understood.
What Are Decentralized Clinical Trials?
Decentralized clinical trials use technology to bring trials beyond the walls of academic medical centers and into doctors’ offices, pharmacies, and even patients’ homes.
In 2019, Scott Gottlieb, M.D., then-Commissioner of the FDA, stated, “We see great potential in using digital technologies to bring clinical trials to the patient, rather than always requiring the patient to travel to the investigator. This is an FDA priority.”
Here are five things you need to know about decentralized clinical trials:
1. The term “decentralized clinical trials” has multiple meanings.
When we talk with sponsors, CROs, and sites about decentralized clinical trials, we often find their definitions of DCTs fall on a spectrum. The four most common types of trials we hear about are:
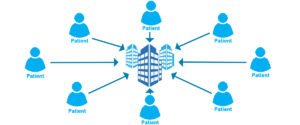
Centralized or Traditional Clinical Trial: Researchers collect data from patients in a centralized location (usually an academic medical center).
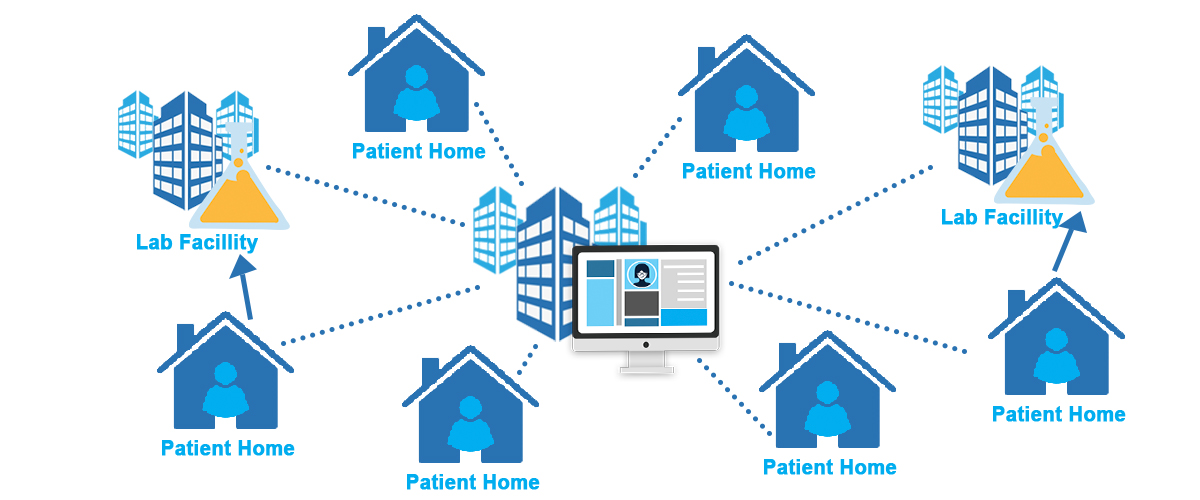
Decentralized Clinical Trial (DCT): Researchers conduct a clinical trial using a variety of methods. They can enroll, consent, monitor, and collect data from patients in their homes using digital tools. They can also deploy ambulatory care teams and may direct participants to regional offices or central labs for infusions and tests.
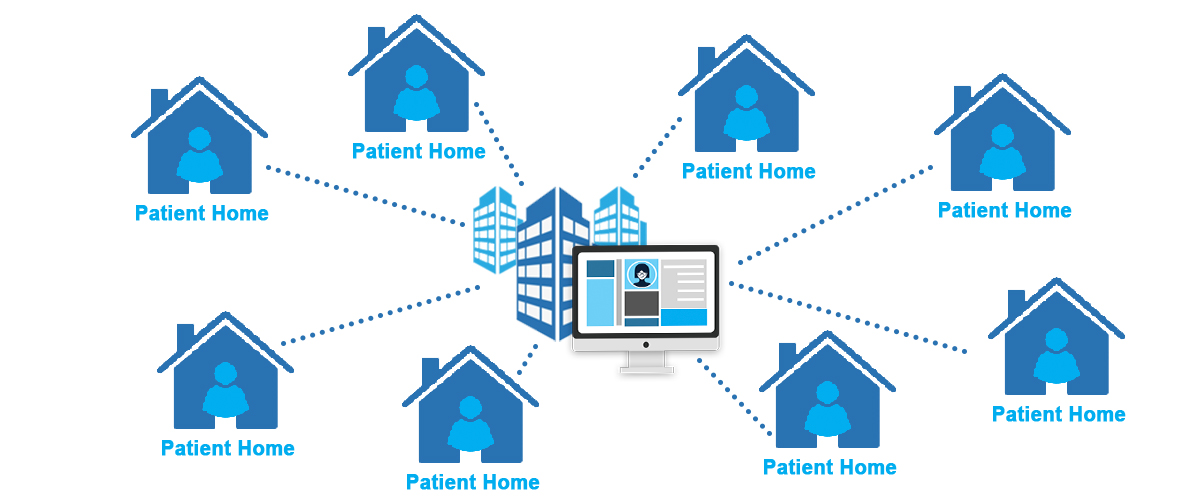
Virtual Clinical Trial (VCT): VCTs are a subset of decentralized trials that are fully virtual. Patients rely on digital tools and devices and have no in-person interactions with researchers. Medical devices, eClinical solutions, and video visits are commonly deployed to facilitate VCTs.
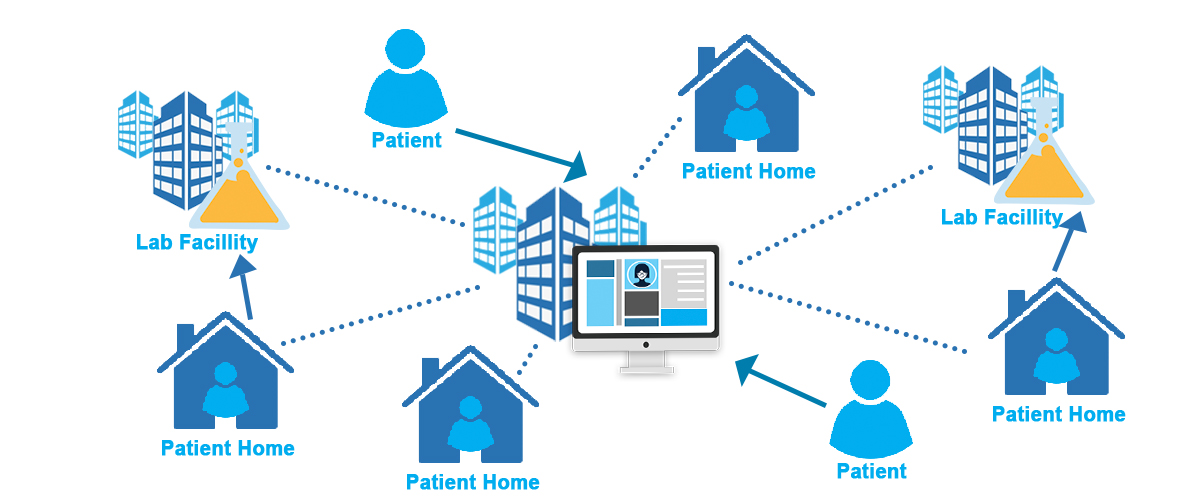
Hybrid Clinical Trial (HCT): In hybrid trials, researchers combine strategies from decentralized and traditional trials to enroll, monitor, and collect data from patients.
The above definitions were first outlined by Florence COO, Angela Gill Nelms, in Clinical Research News Online.
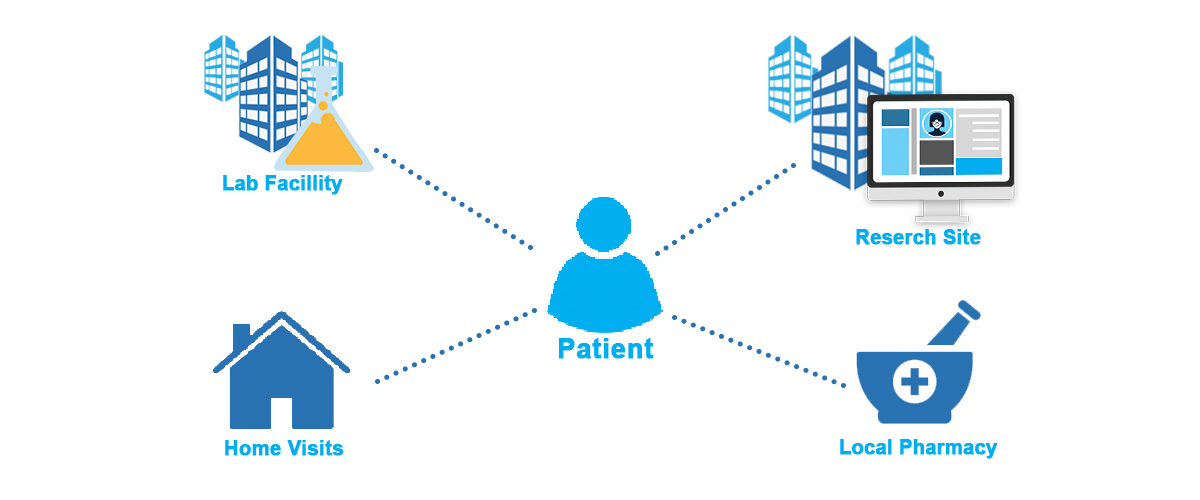
2. Decentralization doesn’t mean site-less. It means localization.
We have over 10,000 study sites in our network, and when discussing decentralized research with them, the first question that always comes up is, “Does that mean the sponsor will bypass us?”
The answer is no. Instead, decentralized operations allow more local and community-based health clinics and provider offices to participate in research.
Researchers can now conduct trial operations at the local pharmacy, at the neighborhood pediatrician’s office, and even through home nurse visits. Decentralized trials help patients who do not traditionally have the opportunity to participate in research by bringing research to them.
The Avoca Group agrees that decentralized trials are more patient-friendly, stating, “Decentralized clinical trials (DCTs) offer a more patient-centric approach, reflecting a transformational philosophy for the conduct of clinical trials in which fewer clinic visits are required and patient and caregiver burden is reduced.”
3. Ease-of-use and user experience matter, a lot.
Traditional clinical trial systems can be hard to use and understand even for highly trained medical professionals. Imagine a patient with a life-changing disease or a small site without staff trying to navigate an EDC or ePro program. It isn’t practical.
But decentralized trial technology is designed for patients, local medical professionals who aren’t familiar with enterprise software solutions, and smaller study sites that don’t have any staff.
For research sites and sponsors to run DCTs successfully, they must embrace technology that’s easy-to-use, simple-to-deploy, intuitive, and flexible enough to adapt to the user’s needs.
4. Without interoperability of technology systems, decentralized clinical trials will fail.
Technology systems that don’t integrate with other platforms won't support decentralized clinical trials. Why? A software platform that's either not designed for research or forces organizations to only use one vendor will lead to duplicate work, time-consuming processes, and unfavorable outcomes.
Former FDA Commissioner Scott Gottlieb, M.D. said that investigators need to “develop new incentives that reward collaboration and data sharing across the clinical research enterprise.”
Without interoperability of technology systems, the FDA goal of collaboration and data sharing becomes difficult or impossible to achieve. Technology systems need to integrate with one another in order to share data.
Anytime you investigate new technology, ask the vendor about its integration and interoperability capabilities. Don't let a vendor make excuses for avoiding integrations – doing so only benefits them.
An open API makes integrations possible by letting you connect one piece of open-API software with any other open-API software. If you'd like to learn more about open APIs, this article offers a great place to start.
5. Clinical operations efficiency and patient experience must be equally important.
Patients often sign up for clinical trials because they want to spend more facetime with their physician or gain access to other experts.
In the words of the NIH, “Healthy volunteers say they participate to help others and to contribute to moving science forward. Participants with an illness or disease also participate to help others, but also to possibly receive the newest treatment and to have the additional care and attention from the clinical trial staff.”
Despite the ability to decentralize many operations, patients still expect interaction with their providers and their research team.
A sponsor, CRO, or study site should be striving to maximize the efficacy of their trial while simultaneously enhancing the patient experience when using decentralized trial technology. One is a recipe for failure without the other.
What's Next?
If you want to know more about preparing for the future of decentralized trials, check out our eBook on decentralized clinical trials for research sites.
About the Author:


