Clinical Trial Diversity: Lessons Learned from a Pandemic
Sites and sponsors struggled with clinical trial diversity long before the COVID-19 pandemic began. Roughly 20% of new drugs have different effects depending on a person’s race. This makes it vital for new treatments to be tested on a diverse patient population.
Yet African American, Hispanic, elderly, and disabled patients are consistently underrepresented in clinical trials in the U.S. The U.K., another major location for clinical trials, also sees consistent underrepresentation of Black, Asian, and South Asian patients.
The COVID-19 pandemic brought both wins and losses for clinical trial diversity. On one hand, many trials halted when the pandemic began. Even once they resumed, they struggled with recruitment, including recruitment of underrepresented patients.
On the other hand, Pfizer and Moderna’s COVID-19 vaccine trials saw greater diversity than average for the clinical trial industry. They achieved this diversity by working with a large network of community-based research sites around the world.
Research sites and sponsors must continue to fight for clinical trial diversity. And to do that, they must learn from the COVID-19 pandemic.
We’ve broken down how the pandemic impacted trial diversity and the vital lessons your clinical trial organization can take away about how to serve underrepresented patients.
The Struggle to Achieve Clinical Trial Diversity
Clinical trials have consistently struggled to recruit participants who are diverse in age and race. 60% of vaccine trials in the U.S. between 2011 and 2020 didn’t include any patients over 65. But 16% of the U.S. population is 65+, and many of the vaccines tested are recommended for that population.
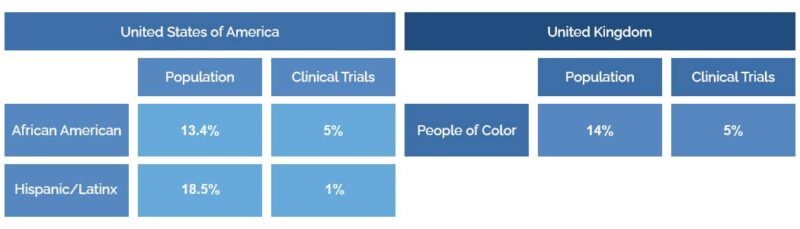
Clinical trials also often fail to have sufficient racial diversity. In the U.S., African American people make up 13.4% of the population and only 5% of clinical trial participants. Hispanic/Latinx people make up 18.5% of the population and only 1% of clinical trial participants.
The numbers are also worrying in the U.K., with people of color making up 14% of the U.K. population but only 5% of medical research participants.
There are many reasons for this lack of diversity. Historical abuses, like the Tuskeegee syphilis study and the theft of Henrietta Lacks’s cells, cause many people of color to justifiably distrust medical research. 53% of patients fear clinical researchers will discriminate against them because of their race or ethnicity.
A lack of diversity among researchers themselves can also lead to a lack of diversity among participants. 40% of participants would like to hear from a researcher that shares their background, but many cannot.
Finally, many underrepresented patients don’t have access to clinical trials. 70% of the U.S. population lives more than 2 hours away from an academic medical center. This makes it difficult for elderly people, people with disabilities, and people who work hourly jobs to join trials.
And then the COVID-19 pandemic hit, causing a dramatic decrease in the number of clinical trials and in participant recruitment rates.
The Challenges COVID-19 Presented for Trial Diversity
From February to May 2020, the number of clinical trials initiated in the U.S. dropped by 43%. Outside of the U.S., the number of clinical trials initiated dropped by 23%.
Ongoing studies were also impacted by the pandemic. The number of completed trials fell by 5.1% in 2020 compared to 2019. The most extreme difference came in the summer. 27.4% fewer trials were completed in July 2020 than in July 2019.
With fewer clinical trials running, fewer patients, including underrepresented patients, had access to new treatments.
Clinical research studies also struggled with participant enrollment because of COVID-19:
- 20% of cancer patients said they were less likely to enroll in a clinical trial than they were before the pandemic.
- 60% of research sites said they were having challenges with patient recruitment since the pandemic began.
Many of the challenges the pandemic created for patients were even more difficult for underrepresented patients. For example, patients over 65 were at high risk for complications from COVID-19, which made enrolling in any trial more difficult.
People who were already battling an illness also hesitated to risk exposure to the virus by traveling to academic medical centers. And many patients who may have been willing to enroll struggled to do so because of travel restrictions.
Maintaining (and Increasing) Diversity During COVID-19 Vaccine Studies
Yet in spite of the recruitment challenges the pandemic created, Moderna and Pfizer’s Phase III COVID-19 vaccine trials managed to be more diverse than vaccine clinical trials are on average.
Comparison: Representation of Underserved Patients in Clinical Trials
| Pfizer vaccine trials | Moderna vaccine trials | Average U.S. trials | |
| African American/Black participants | 9.3% | 10.2% | 5% |
| Asian American participants | 4.3% | 4.6% | 5.7% |
| Hispanic/Latinx participants | 28% | 20.5% | 1% |
| Older participants | 41% (56-85; Pfizer and Moderna bracketed age groups differently) | 25% (65+; Pfizer and Moderna bracketed age groups differently) | 21% (65+) |
Although representation for Asian American participants decreased slightly during the COVD-19 vaccine trials, representation of African American, Hispanic, and elderly participants increased.
So in a time when patient recruitment was steadily dropping, how did Pfizer and Moderna manage to recruit more diverse patients than the typical clinical trial? There were two key factors:
- Public awareness
- A network of community sites
The COVID-19 vaccine trials were more highly publicized than the average clinical trial. But The Lancet also credits Pfizer and Moderna’s large network of community clinical trial sites for their recruiting success.
And while every trial can’t replicate the publicity of the COVID trials, every trial can build a network of community sites to meet diverse patients where they are.
How Building a Strong Site Network Increases Clinical Trial Diversity
70% of the U.S. population lives more than two hours away from an academic medical center. This long travel time often presents difficulties for:
- Older people
- People with disabilities
- People with hourly jobs
- People who rely on public transportation
Dr. Hala Borno, an expert in building diversity in clinical trials, advocates for clinical trials taking place at community sites in underserved areas, instead of only at academic medical centers in major cities.
Smaller community sites often don’t have the technology or personnel to run trials on their own–but they can do so with regulatory and technology help from sponsors, CROs, and coordinating centers (we have more info for coordinating centers here!)
For example, a coordinating center could send online study start-up kits with binder and folder setups and document templates to community sites. The community sites could receive funding and experience from getting involved in research, and the coordinating center could gain access to a more diverse participant population.
This approach has worked for multiple clinical trials, and it proved invaluable during the pandemic.
Success in Using Community Sites for Clinical Trial Diversity
When confronted with how to recruit diverse clinical trial participants in spite of a pandemic, clinical trial organizations turned to past success stories, like the SPRINT blood pressure trial. This trial, run by Dr. Thomas Ramsey, worked with 19 coordinating centers across five different health networks.
The SPRINT trial saw the following results:
- 102 participating community sites
- 15,000 participants (more than the 9,000 minimum for trial)
- 50% of participants from racial and ethnic minorities
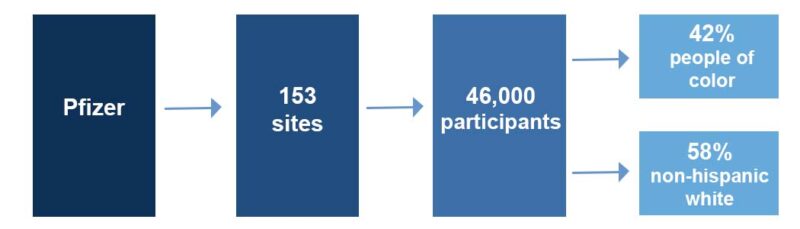
Pfizer used similar methods to ensure diversity in their COVID-19 vaccine trials. They reached out to 153 clinical trial sites across the U.S., Argentina, Brazil, South Africa, Turkey, and Germany.
These local sites were able to enroll more than 46,000 participants, and approximately 42% were Asian, Black, Hispanic/Latinx, or Indigenous/Native American, with only 58% being non-Hispanic white.
Though these numbers don’t fully represent the global population, they come much closer than clinical trials typically do, providing evidence that local site networks are effective.
How Technology Helped Maintain Clinical Trial Diversity
In order to reach community sites and diverse patients around the world, Pfizer and other sponsors needed technology that connected them to their sites.
Monitors needed to see documents and data instantly even when travel was restricted, and site staff needed training and to share documents and data with their sponsors remotely.
Technology helped with the following aspects of clinical trials during the pandemic:
- Speeding up communicaton
- Providing reports on site progress
- Collecting data from participants
- Training site staff
Speeding Up Communication
Remote monitoring makes it easier for sponsors to introduce sites to the concept of decentralized trials and help sites use new technology. Smaller sites, especially community sites that care for underserved patients, often can’t afford the eISFs or electronic participant binders that enable remote monitoring.
But if sponsors pay for the technology while letting sites retain ownership of it, as Pfizer did during the vaccine trials, those small community sites can become involved in large global trials. In turn, sponsors can benefit from the diverse participant populations those sites work with.
Remote technology also helped non-patient-facing staff, who worked at home during the pandemic, communicate with patient-facing staff who were still at the site, like nurses and investigators. Without this communication, more clinical trials could have been halted or slowed down.

Providing Reports on Site Progress
With hundreds of sites running COVID-19 studies around the world and travel severely restricted, sponsors and CROs couldn’t wait for monitoring visits to see how studies were progressing.
Technology stepped up to help with this. Monitors could log onto dashboards or view reports within eISFs or electronic participant binders to understand where sites were in a study. This allowed monitors to do their jobs mostly or fully remotely.
In the past, sponsors often relied on document upload portals to track how their sites were doing. But sites resisted adopting technology that added extra tasks to their day and didn’t help them be more efficient. The burden was even greater for small community sites.
That’s why the pandemic brought rapid growth in technology designed to help sites’ workflows.
When sites use an eISF or participant binder to be more efficient and then give their sponsor access to that platform, sites don’t have to do any extra work. This is vital for community-based sites that are often underfunded and understaffed.
Collecting Data from Participants
Technology allows clinical trial sites to collect data straight from participants. This data could include symptoms recorded in electronic patient diaries or data received from sensors and wearable devices.
Though this form of software isn’t common yet, it has potential for engaging participants who are underserved by clinical trials. Participants who work strict hours or don’t have access to a car could submit some of their data from home, which would allow them to visit the research site less often.
Remote Training of Site Staff
Clinical trials require additional training for regulatory staff and physicians. If community sites want to get involved in research, they’ll need training from experienced Clinical Research Coordinators, Clinical Research Associates, and Principal Investigators.
Technology, including video conferencing, webinars, and training videos, can help clinical trial professionals share their expertise with the staff at smaller sites. Those sites can then give their patients access to the latest treatments through clinical trials.
Training and gaining digital research skills can benefit site and sponsor staff members, making them more competitive in their chosen careers.
Learning from the Pandemic to Increase Clinical Trial Diversity
The COVID-19 pandemic could have struck a devastating blow to clincial trial diversity. Many studies were shut down, and even once they began again, participant recruitment became far more difficult.
Yet the COVID-19 vaccine studies proved that it was possible to increase clinical trial diversity, even among less-than-ideal circumstances. Sponsors like Pfizer found diverse participants by expanding their outreach, working with community sites around the world that served underrepresented populations.
Though COVID-19 vaccines are now widely available, the lessons sponsors learned from the pandemic remain relevant. To achieve clinical trial diversity, sponsors need to go to underserved patients, rather than waiting for patients to come to them. And to do that, sponsors and CROs need to embrace remote technology that lets them connect with sites across the globe.
If you’d like to learn more about how decentralized site networks can help clinical trial organizations reach more patients than ever before, check out our 2022 State of Clinical Trial Operations Technology Report, where we cover this movement in detail.
References
Anderson, D., Fox, J., & Elsner, N. (2021). Digital R&D. Deloitte Insights. Retrieved February 11, 2022, from https://www2.deloitte.com/us/en/insights/industry/life-sciences/digital-research-and-development-clinical-strategy.html
Ending the ‘diversity gap’ in research: Shadow Science Minister joins pharma experts and BAME leaders to take action. (2021, November 9). Innovative Trials. Retrieved February 11, 2022, from https://innovativetrials.com/press-release-ending-the-diversity-gap-in-research/.
Feuerstein, A., Garde, D., & Robbins, R. (2020, August 14). Covid-19 clinical trials are failing to enroll diverse populations, despite awareness efforts. STAT. Retrieved February 11, 2022, from https://www.statnews.com/2020/08/14/covid-19-clinical-trials-are-are-failing-to-enroll-diverse-populations-despite-awareness-efforts/
Flores, L. E., Frontera, W. R., Andrasik, M. P., del Rio, C., Mondríguez-González, A., Price, S. A., Krantz, E. M., Pergam, S. A., & Silver, J. K. (2021). Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Network Open, 4(2). https://doi.org/10.1001/jamanetworkopen.2020.37640
Le Breton, S., Lamberti, M. J., Dion, A., & Getz, K. A. (2020, October 22). Covid-19 and its impact on the future of clinical trial execution. Applied Clinical Trials Online. Retrieved February 11, 2022, from https://www.appliedclinicaltrialsonline.com/view/covid-19-and-its-impact-on-the-future-of-clinical-trial-execution
Ledford, H. (2021). The covid pandemic’s lingering impact on clinical trials. Nature, 595(7867), 341–342. https://doi.org/10.1038/d41586-021-01569-9
Mitchell, E. J., Ahmed, K., Breeman, S., Cotton, S., Constable, L., Ferry, G., Goodman, K., Hickey, H., Meakin, G., Mironov, K., Quann, N., Wakefield, N., & McDonald, A. (2020). It is unprecedented: Trial management during the COVID-19 pandemic and beyond. Trials, 21(1). https://doi.org/10.1186/s13063-020-04711-6
National Heart, Lung, and Blood Institute. (2021, June 25). Researchers take cues from the past to ensure diversity in COVID-19 clinical trials. National Heart, Lung, and Blood Institute. Retrieved February 11, 2022, from https://www.nhlbi.nih.gov/news/2021/researchers-take-cues-past-ensure-diversity-covid-19-clinical-trials
Nephew, L. D. (2021). Accountability in clinical trial diversity: The buck stops where? EClinicalMedicine, 36, 100906. https://doi.org/10.1016/j.eclinm.2021.100906
Nishiwaki, S., & Ando, Y. (2021). Covid-19 pandemic and trends in clinical trials: A multi-region and global perspective. Frontiers in Medicine, 8. https://doi.org/10.3389/fmed.2021.812370
Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. (2020, November 18). Pfizer. Retrieved February 9, 2022, from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine.
Pfizer. (2022). About our landmark trial. Pfizer. Retrieved February 11, 2022, from https://www.pfizer.com/science/coronavirus/vaccine/about-our-landmark-trial
Ramamoorthy, A., Pacanowski, M. A., Bull, J., & Zhang, L. (2015). Racial/ethnic differences in drug disposition and response: Review of recently approved drugs. Clinical Pharmacology & Therapeutics, 97(3), 263–273. https://doi.org/10.1002/cpt.61
Yates, I., Byrne, J., Donahue, S., McCarty, L., & Mathews, A. (2020, August 11). Representation in clinical trials: A review on reaching underrepresented populations in research. ACRP. Retrieved February 11, 2022, from https://acrpnet.org/2020/08/10/representation-in-clinical-trials-a-review-on-reaching-underrepresented-populations-in-research/
Portions of featured image from https://www.freepik.com/vectors/background
Background vector created by rawpixel.com – www.freepik.com

