Free Download
Complete Guide to eISF, SDR/V +
Remote Site Access in Clinical Trials
How clinical operations leaders leverage remote site access technology to enable remote monitoring, start-up, management, and SDR/V.
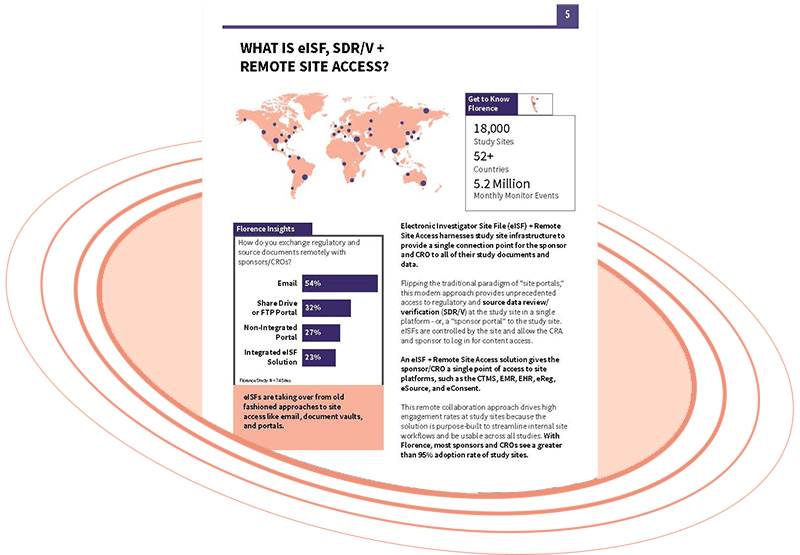
You need remote access to your study sites, and you need your strategy to not fail. After working with over 18,000 study sites across 70+ countries to implement eISF + Remote Site Access platforms, we know the secrets to how Sponsors/CROs can enable true remote site access, monitoring, and SDV/R at their study sites.
This guide will show you best practices, how-tos, and insights for implementing your own remote site access strategy: what is the eISF and how does it enable remote site access, how you can select a platform that will work for you and your sites, how to maximize the benefits, and how to get your study sites on board.