How Digital Connections Can Create a Positive Patient Experience in Clinical Trials
Patient experience in clinical trials is a problem. On patient satisfaction surveys, clinical trials received low scores similar to the scores of Internet service providers, utility companies, and health insurers–businesses infamous for poor client experiences.
A positive clinical trial experience encourages patients to participate in future trials and to recommend trials to their loved ones. Since clinical trials often struggle with recruiting patients, retaining participants, and building strong public reputations, sites, sponsors, and CROs need to focus on patient experience for their trials to succeed.
Digital clinical trials can improve the patient experience by bringing studies to local sites and patients’ homes. With technology, sponsors, CROs, and sites can quickly communicate with one another across states or countries, and participants can spend less time traveling–a major source of patient dissatisfaction.
In this article, we’ll cover:
- What is “patient experience” in clinical trials?
- Problems with the patient experience in clinical trials
- 4 ways digital connections can create a positive patient experience in clinical trials
Keep reading to learn how you can build an amazing patient experience during your clinical trials.
What Is “Patient Experience” in Clinical Trials?
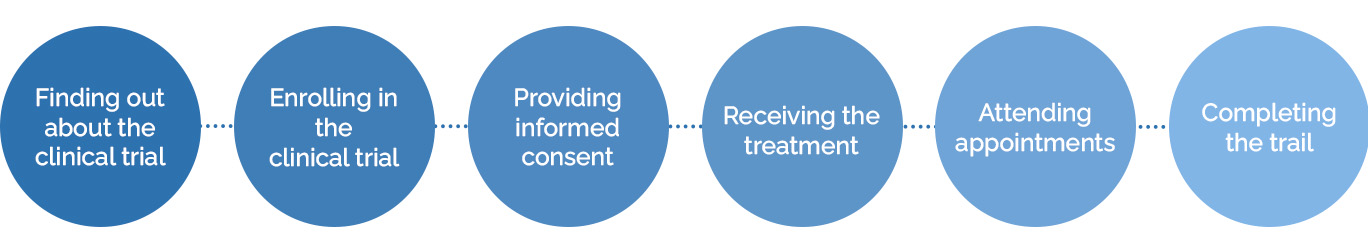
The patient experience in clinical trials is how patients feel about a trial, from the time they find out a study exists to the time they complete it.
The patient experience includes:
- Finding out about the clinical trial
- Enrolling in the trial
- Providing informed consent
- Receiving the treatment
- Attending appointments
- Completing the trial
- Participating in post-trial activities, like follow-up, surveys, or receiving results
Patient experience can be measured using net promoter scores (NPS), a common scale for figuring out customer satisfaction. On this scale, patients say whether they would recommend clinical trials to a loved one.
Unfortunately, clinical trials often receive low NPS scores–and that’s when trials choose to evaluate patient experience. But why do these low scores happen?
Problems with Patient Experience
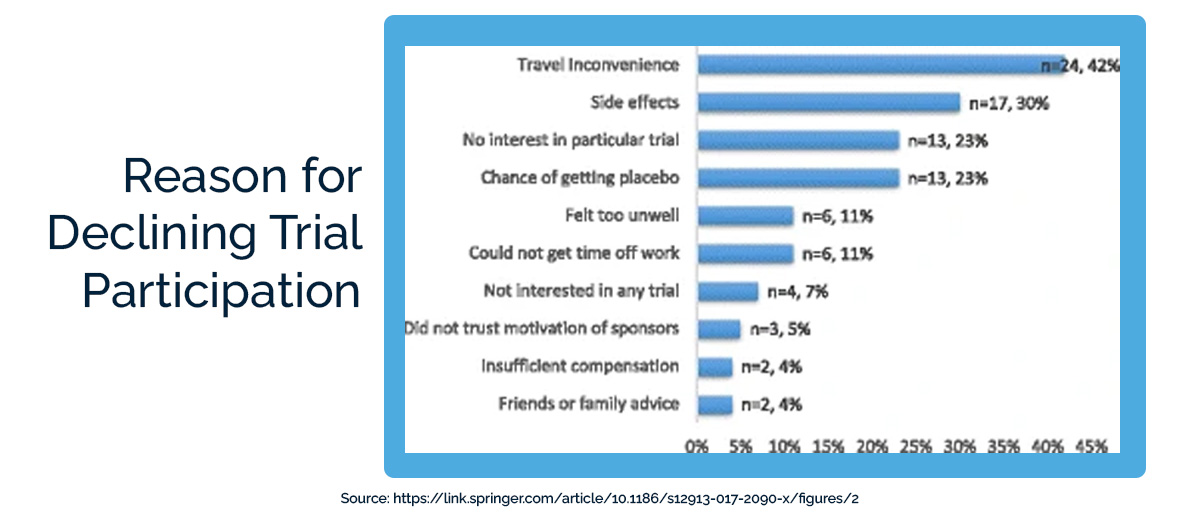
In one large survey, 20% of participants said they were “slightly” or “not at all” satisfied with their clinical trial experience. 58% were “extremely” or “very satisfied.”
While it’s encouraging that 58% of participants had an overwhelmingly positive experience, it’s worrying that 42% saw substantial room for improvement. A majority of participants with lupus, epilepsy, rheumatoid arthritis, and fibromyalgia said that they would tell other patients with their condition not to join clinical trials.
When participants don’t recommend trials to other patients, it creates problems for sites and sponsors:
- 45% of UK sites need an extension to meet their recruitment goals
- 80% of U.S. sites need an extension to meet their recruitment goals
- Recruitment delays can cost sponsors $600K-$8M per day
So why are patients either not joining clinical trials or telling others not to join clinical trials? The number-one reason they gave was the inconvenience of travel (42%). Potential participants worry even more about travel than about side effects or receiving a placebo.
The good news? With the rise of technology and decentralized clinical trials, lengthy travel to large clinical trial sites is no longer necessary. Participants can now take part in trials at local sites and even from home.
Technology also helps participants submit feedback on trials and helps sites and sponsors try new, more patient-centric trial designs.
4 Ways Digital Connections Can Create a Positive Patient Experience
Digitally-enabled clinical trials can improve the patient experience by:
- Involving local sites so participants don’t have to travel long distances
- Connecting sponsors and sites to make decentralized trials possible
- Letting participants submit some data from home
- Giving patients input into trial design
Here’s how each of these changes contributes to more effective, patient-centric clinical trials:
1. Involving local sites so patients don’t have to travel long distances
Clinical trials that involve local doctor’s offices, pharmacies, and clinics can recruit more patients, more quickly.
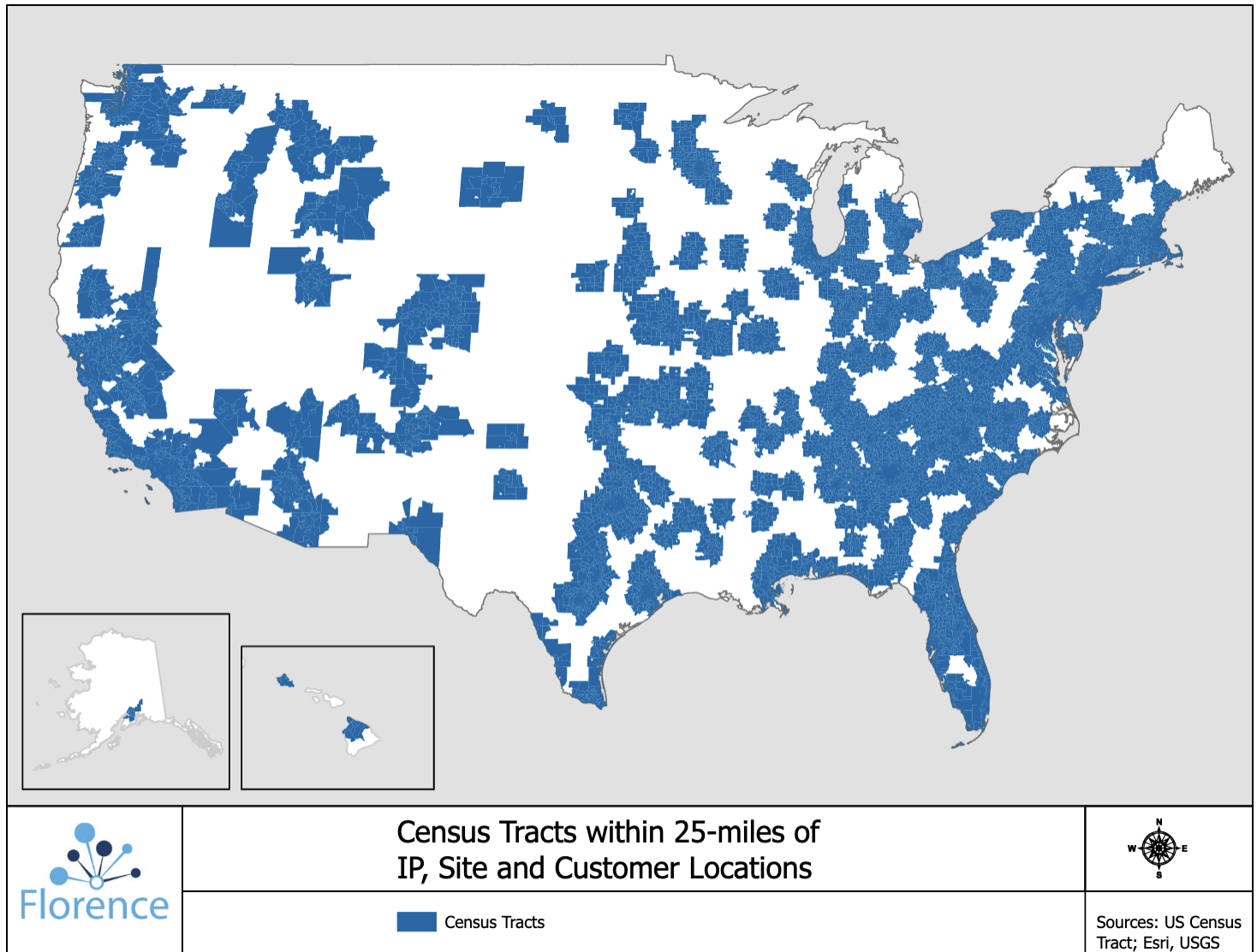
Only 30% of patients live within 2 hours of a major academic medical center (AMC). But 80% of patients live within 25 miles of a research site that uses Florence’s technology. Technology lets smaller sites collaborate with larger AMCs, who can help them set up trials and manage regulatory documents.
Involving local sites is a win for sponsors, who have access to larger patient populations, and for patients, who can visit sites within their own neighborhood instead of traveling hours to a cancer center or AMC.
Patient advocacy groups rank transportation to trial visits and out-of-pocket travel expenses as major recruitment barriers. Even if sponsors agree to cover transportation costs, traveling can be difficult for participants who work, care for children, or have a serious illness or disability.
Visiting sites in their own neighborhood could greatly reduce these barriers and improve patients’ experience of clinical trials.
2. Connecting sponsors and sites to make decentralized trials possible
When clinical trial organizations talk about decentralized clinical trials (DCTs), they often focus on using technology so sponsors or sites can connect with patients. This is important, but it overlooks another critical component of digital trials: digital clinical trials let sites and sponsors connect to each other.
We cover how connecting sites and sponsors allows DCTs to happen in this article. But how does this improve patient experience?
When sponsors can collect regulatory documents and patient data remotely, they can engage with local community sites near more underrepresented patients. They will no longer have to worry about sending Clinical Research Associates (CRAs) in person for every monitoring visit, so they won’t have to limit the number or location of their study sites as strictly.
This lets far more communities get involved in clinical trials–especially communities of historically underserved patients.
3. Letting participants submit some data from home
Decentralized clinical trials don’t always mean direct-to-patient trials. Especially when dealing with complex illnesses, patients will need to visit the site in-person for treatments, scans, and tests at least some of the time.
But that doesn’t mean patients can never submit data from home. With digital technology like electronic Patient-Reported Outcome (ePRO) software, electronic patient diaries, and telehealth calls, participants can report symptoms and experiences from home instead of only during site visits.
Sites can then use in-person visits for important treatments and building a relationship with patients, instead of for quick, routine check-ins. This lets participants travel to the site far less frequently.
For example, participants often have a visit with clinical trial staff to discuss the informed consent document. They then either have to sign the paper form right away or go home, talk with their family, and return to the site to finish the form.
With eConsent, participants can review the form on their phone or tablet, show it to their loved ones, and send it straight back to the site–with no travel required.
In one study of patients with rare diseases, 60% of them liked that decentralized trials could mean less time traveling. Since travel is one of the largest causes of dissatisfaction with trials, combining local site visits with digital data submission will improve patient experience substantially.
4. Giving patients input into trial design
One often-overlooked way to improve patient experience in clinical trials: let participants help design the trials.
Sponsors and CROs can invite patient advisory groups, patients, and caregivers to initial protocol creation sessions. They can also seek feedback from participants throughout the trial process that they can incorporate into future trials.
In one study of cancer trials in the U.K., studies that included patient input were twice as likely to hit their recruitment targets. Advocacy groups may also help with recruitment awareness campaigns if they feel clinical trial sponsors care about their experiences.
Improving patient experience in clinical trials requires making trials patient-centric from the moment they’re conceived and collecting feedback from patients until the end.

Improving the Patient Experience in Clinical Trials
When patients have a positive experience with clinical trials, they won’t just participate in one trial–they’ll return for more studies and encourage their loved ones to join studies too.
Clinical trial participants frequently report travel and the time spent on in-person appointments as reasons they have negative experiences. With all of the decentralized technology now available, sponsors can utilize local sites and at-home data reporting to cut down on the need for travel. This change alone will go a long way toward improving patients’ clinical trial experiences.
To learn more about the power of links between sponsors and local sites, check out our article on the future of community-based clinical trials.

