How Community-Based Clinical Trials Benefit Sponsors, Local Healthcare Systems, and Patients
Clinical trials could help far more patients than they currently do. So how do we expand studies to all of the patients who need them? Community-based clinical trials might be the answer.
70% of patients live over two hours from the closest academic medical center (AMC), and many groups of patients are underrepresented within trials. Community-based trials address this problem by holding trials at local, smaller sites instead of only at large academic medical centers (AMCs). Patients can visit their personal physician or their closest pharmacy in between visits to the main site.
With community-based trials, sponsors and CROs can reach new patients and speed up their recruitment efforts. In turn, local communities will see economic benefits and more options for healthcare, including new and potentially life-saving treatments.
Keep reading to learn how to make community-based clinical trials a reality in your neighborhood!
The Benefits of Community-Based Clinical Trials for Sponsors
Community-based clinical trials offer tremendous value to both trial sponsors and local communities.
For sponsors, community-based clinical trials can help with diversity and recruitment, two areas where trials often struggle. Black, Indigenous, and Hispanic people are consistently underrepresented in clinical trials, as are elderly and LGBTQ+ people.
Since roughly 20% of new drugs have different effects on people of different races, sponsors must recruit diverse clinical trial participants to ensure that their treatments work.
But recruitment takes time. 80% of trials experience delays because of recruiting difficulties, and 85% of clinical trials fail to hit their recruitment goals. If sponsors want to accelerate their recruitment timelines and ensure their trials represent the population, they need to reach the 70% of patients who don’t live near major academic medical centers.
There are many reasons patients hesitate to participate in trials. They may:
- Not be able to take off work
- Not be able to afford childcare for long periods of time
- Not have a car and rely on public transportation
- Not feel healthy enough to travel long distances
Community-based clinical trials can eliminate many of these challenges. Participants can visit a local pharmacy, doctor’s office, or lab instead of driving hours to visit the main site. This means they don’t have to take an entire day off work and may be able to walk or take transit to their destination.
In one study of patients living with rare diseases, 60% of patients liked that decentralized trials helped them spend less time traveling. This is a major win for sponsors looking to increase recruitment of underrepresented patients.
Bringing Trials to Communities Where Patients Live
Technology can help sponsors bring trials to where patients are. For example, 70% of U.S. patients live more than 2 hours from an academic research center. But 80% of U.S. patients live within 25 miles–about 30-45 minutes–of a clinical research site that uses Florence’s tech.
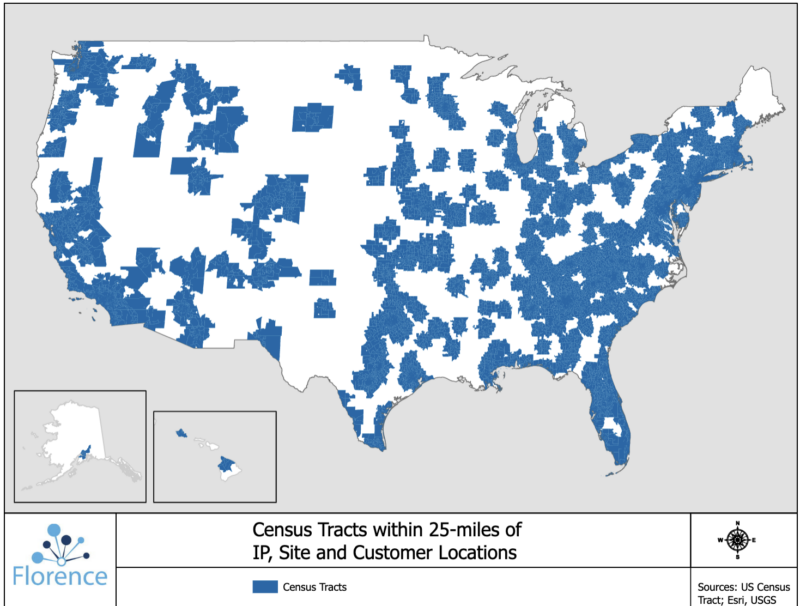
How is this possible? Florence is in use not only at major academic medical centers but also at thousands of smaller research sites. By working with these sites, sponsors can reach about 285 million potential participants for studies.
But working with these smaller sites requires moving beyond major cities. That’s why sponsors, CROs, and coordinating centers need to connect to sites across the U.S. or multiple countries using technology. With remote access technology, sponsors can:
- Distribute regulatory documents to smaller sites
- Monitor study documents and data remotely
- Provide regulatory help to staff who aren’t familiar with clinical trials
Whether sponsors opt for Florence or a different remote access platform, connecting to smaller sites outside of major cities is a crucial step in bringing trials to more patients than ever before.
The Benefit of Community-Based Clinical Trials for Local Health Systems
Clinical trials offer new treatment options that many underrepresented patients have previously lacked.
Andrea Bastek, Florence’s Senior Director of Innovation, points out that many patients in rural or underserved areas “look to leaders in their community or their long-time local physicians for advice about illness, therapy options, and clinical trials.”
By participating in trials, health systems and physicians in underserved areas can give their patients more options for healthcare, including cutting-edge treatments.
So why don’t more community sites currently participate in trials? Smaller sites often face funding and staffing challenges that are less prominent for larger AMCs or cancer centers. But technology and sponsors can help alleviate these challenges.
Getting Local Sites Involved in Community-Based Trials
Smaller community sites might worry that they don’t have the infrastructure to support trials. Although sponsors pay for studies, those payments often don’t cover initial start-up expenses, like hiring regulatory staff or equipping trial facilities.
One solution? Sponsors can form working relationships with health systems that serve underrepresented patients. CROs or sponsors could then fund the initial infrastructure for studies. In return, they’ll have larger patient pools to draw from for their trials.
This strategy has already yielded results for many local communities. A 2019 TEConomy Partners report found that sponsors’ investments in clinical trials generated $27.4 billion in additional revenue for the communities where trials took place.
CROs and sponsors can also help sites that don’t have an abundance of regulatory staff. Local sites need software that’s easy to use, so it’s important that CROs or sponsors:
- Provide intuitive, site-friendly eISFs
- Distribute templates for electronic documents and folders
- Offer continuous remote monitoring so they can help identify missing or incomplete documents
Sites that don’t have a strong relationship with sponsors or CROs don’t have to be cut out of clinical trials, though. Research networks, such as provider-based research networks (PBRNs), can also help sites get involved in community-based clinical trials.
Building Community-Based Trials with Research Networks
Sponsors are a critical source of funding for clinical trials, but not the only one. Governments and philanthropic organizations also fund a large percentage of trials, and they can also reach more patients through local sites.
For example, the National Cancer Institute sponsors the Community Oncology Research Program (NCORP), which works with more than 1,000 community clinics and physicians across the U.S.
When small sites join an organization like NCORP, they receive federal funding to cover the expenses of investigator-initiated studies. They can also benefit from the help of larger research centers within the network.
Academic research hospitals can serve as coordinating centers, using remote access to distribute online folders and document templates to sites. Their staff can also share their expertise with community sites.
Sites that have never run a clinical trial before can turn to coordinating centers for help with completing regulatory documents, following Good Clinical Practice guidelines, and uploading data. In turn, the entire site network can benefit from reaching more communities and more patients.
How Community-Based Clinical Trials Can Help Sponsors and Health Systems
By working with local communities, sponsors can gain access to a new pool of participants who are waiting for cutting-edge treatments. Local health systems can also reap the benefits of funding and expertise flowing into their communities.
But the most important benefit of community-based clinical trials is that patients have more options for their healthcare. By embracing community-based clinical trials, small sites and sponsors can give patients, including underrepresented patients, treatment options they’ve never experienced before.
To learn more about how community-based site networks can help with patient diversity, check out our blog on how sites stepped up their inclusion efforts during the COVID-19 pandemic.

