See how it works

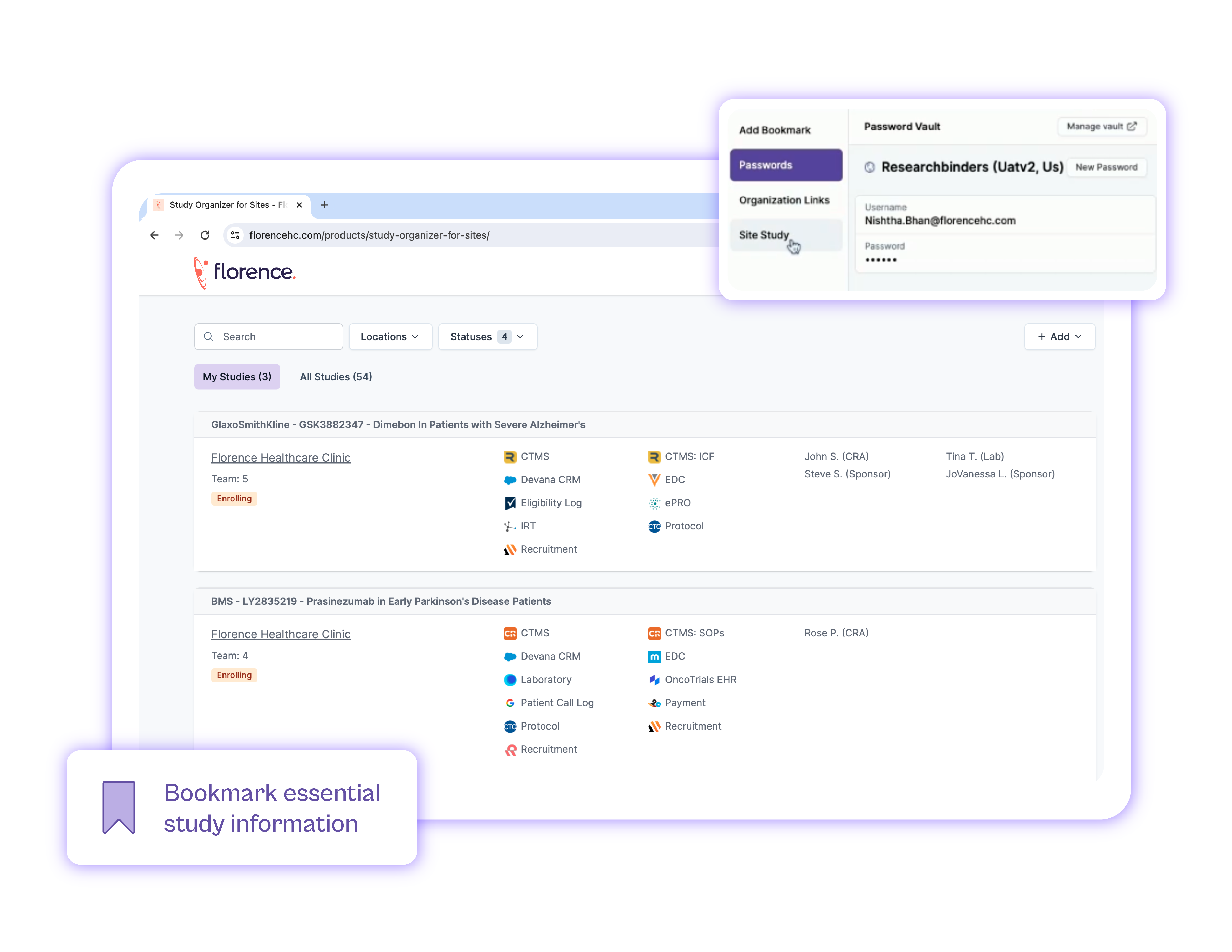
Bookmarks
All of your shortcuts in one place, organized to the right study.

Contacts
Manage all of your study contacts both internally and externally.
Sticky Notes
Track key details like eligibility, amendment info, and patient prep notes.
Password Vault
Bulk import credentials and ensure smooth team access.
Empowering sites, sponsors & CROs
Keep all study contacts, systems, passwords, and key details in one place for easy access.
Provide a safe location for your team to store and manage all passwords for study systems.
Connect with CTMS and other systems to streamline the creation of study profiles and information.
Quickly build your tool library through crowdsourcing of technologies used across sites and studies.
Sites can set up in minutes and begin using StudyOrganizer the same day.
A complimentary solution to ensure sites have access to top-tier technology.
Pre-load study tools, provide access details, and centralize essential study information to streamline onboarding, tech adoption, and continuous staff training.
Use pulse surveys to identify blockers early, improve site engagement, and ensure smooth operations.
Facilitate streamlined communication with a centralized platform and easily extend the solution to multiple studies, ensuring your site is always prepared and efficient.
Integrate with the tools you use every day.
In clinical research, compliance is crucial. We’ve got you covered globally.