What you will learn:
- How remote site connectivity via site-based eISF platforms impacts clinical trial operations.
- How to turn-on remote monitoring at research sites.
- How to turn-on remote SDV at research sites.
- Why connecting to an existing software platform ensures adoption.
- How to leverage a technology partner to get sites on-board fast.
- How to drive TMF quality and completeness with site eISF integrations.
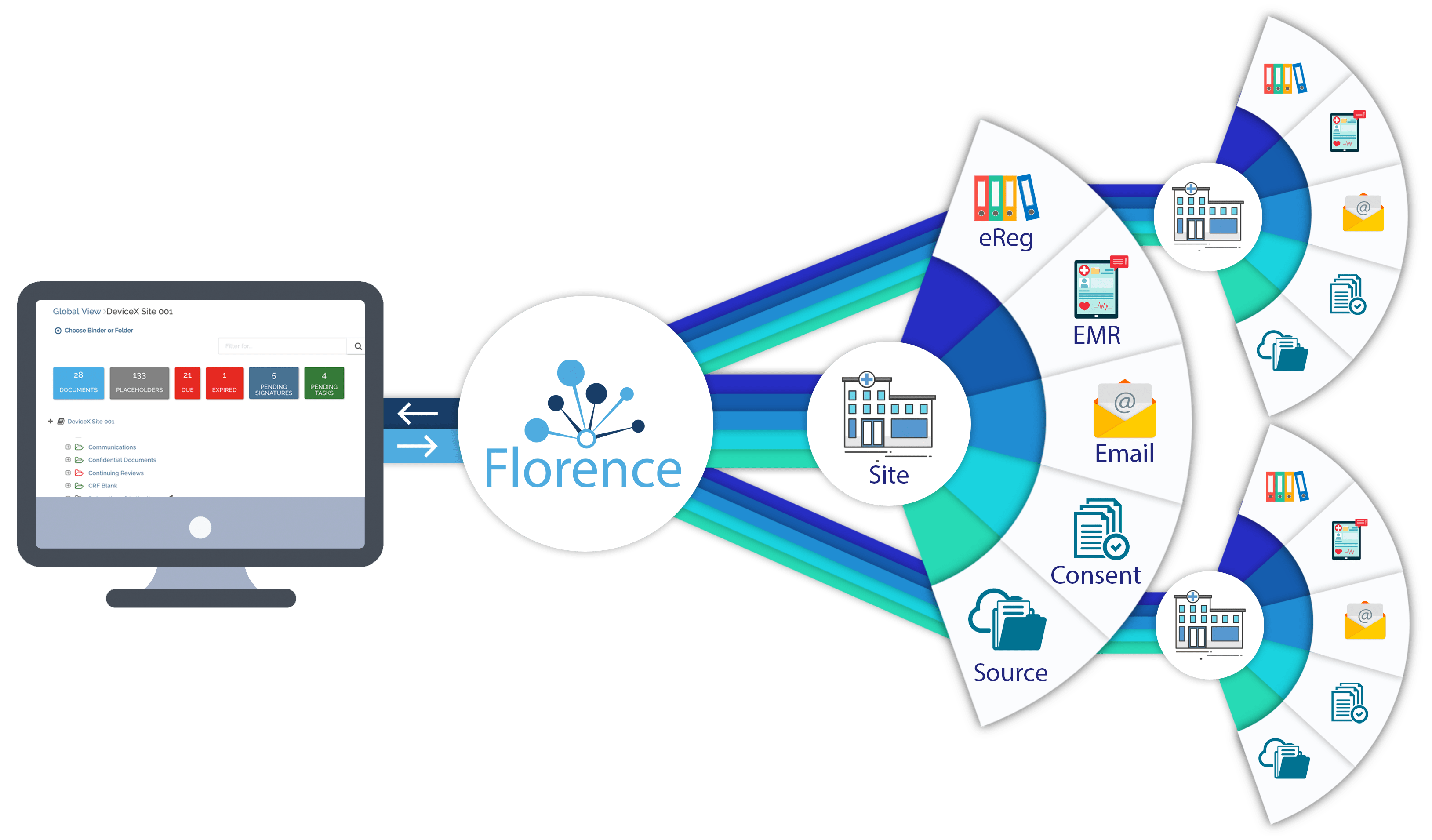
Key Insights Covered
Convergence of site-based eISF solutions enables remote connectivity.
Sponsors and CROs benefit from a trifecta of eISF maturity, a critical mass of 7,200 Investigator sites managing their eISF on the Florence platform, and an urgent focus on remote access.
Remote eISF connectivity transforms core study processes.
Digital site access transforms regulatory document collection, technology set-up, monitoring and SDV, TMF management, and study archival.
Sponsor and CROs accelerate timelines and reduce costs.
Remote site access improves study start-up times by as much as 40%, reduces on-site visits, and reallocates resources from repetitive tasks.
Sponsors and CROs gain real-time site oversight and management.
Because all sites manage eISF documents and workflows in a single, unified, platform grants Sponsors and CROs unprecedented site visibility and management capabilities.

A Sponsor’s and CRO’s ability to access and collaborate with a research site throughout a clinical trial is critical to the study’s success. Every primary study phase and clinical operations process requires the ability to access, review, and exchange documents and data quickly.
A disconnected mix of on-site visits, email communications, and clunky FTP portals restrict Sponsor and CRO study site access and document exchange. Until recently, the lack of site-based technology was the primary reason for the need for this inefficient process.
What has changed? Study sites are making significant investments in purpose-built Electronic Investigator Site File (eISF) solutions to streamline their internal operations, with 63% of sites planning to have a platform in place by the end of 2020.
As study sites “come on-line,” Sponsors and CROs are in a unique position to harness this electronically connected network of sites to turn-on remote site access, monitoring, SDV, oversight, management, collaboration, and document and data exchange.
Florence is on the leading edge of this transformation, currently powering over 7,200 study sites with its eBinders platform for eRegulatory, and is connecting Sponsors and CROs to their study sites via Florence eHub.
Florence eHub is a collaborative workspace, providing unprecedented levels of remote site access, document quality control, and, ultimately, decreasing study cycle times while increasing quality of submissions.
In this whitepaper, we examine core clinical trial processes Florence eHub accelerates.



