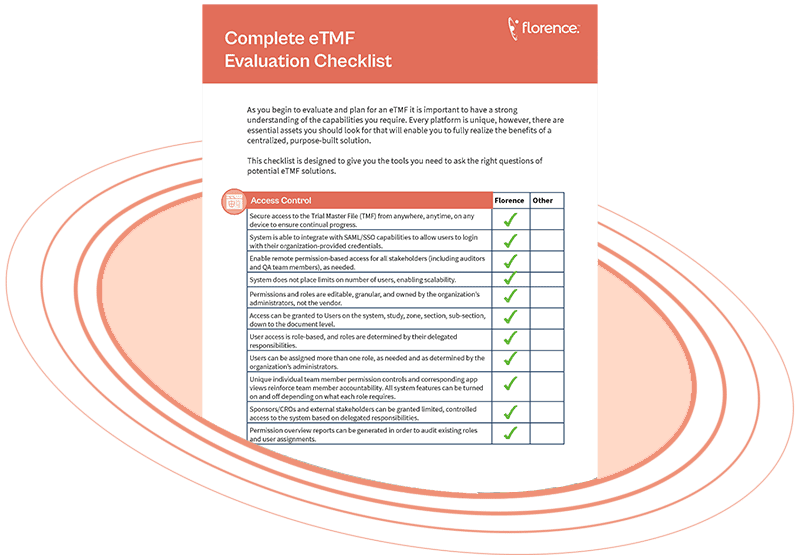
Free Download
The Complete eTMF Capabilities and Evaluation Checklist
This free checklist gives you the tools you need to evaluate potential eTMF solution providers. Get insights compiled from working with 18,000+ sponsors, CROs, and research sites.
You’ll learn what features to look for such as:
- Connecting your eTMF digitally to your study sites eISF
- Maintaining inspection readiness and compliance
- Creating, editing, and maintaining study documents
- Integration with other platforms & more!
Trusted by Sponsors & CROs, Loved by Sites