Meet with Florence at SCOPE Summit 2022
Schedule a 15-minute meeting with our team at Booth 513
Already chaotic workflows for clinical trials get even more chaotic with lockdowns and trial restrictions. Meet with us at SCOPE to learn how to keep studies going during Omicron & other variants. Learn how Pfizer, a top CRO, and top Japanese Pharma keep their clinical trials on track with remote connectivity to their sites.
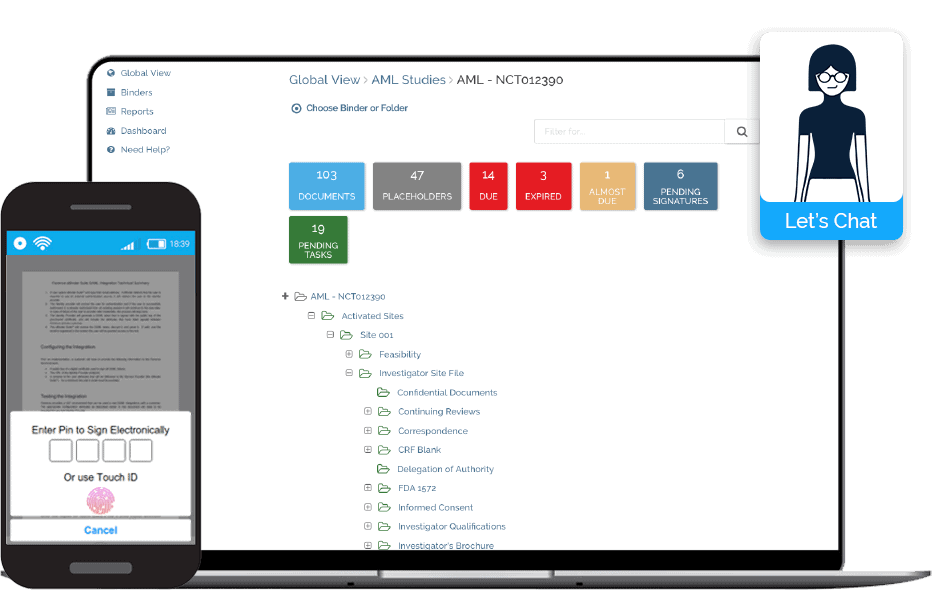
Florence eHub

Florence eHub provides sponsors and CROs real-time remote site access through the industry-leading electronic investigator site file (installed at 10,000 sites) for start-up, monitoring, source data verification, and source data review.
Set up your personal 15-minute demo to see:
- How we get 92%+ of your sites digitized and activated in days.
- How major sponsors and CROs are activating remote monitoring.
- How to turn on remote source data verification and source data review.
- How sponsors are rethinking their protocols and monitoring plans.
- How many of your sites are already on the Florence network (we have 10,000 in 44 countries).