Regulatory Compliance in the Global Clinical Research Evolution
The wheels of globalization have been turning at Florence for more than five years. As a purpose-built clinical trial document, data, and workflow management solution, Florence has its roots at the heart of research – the clinical trial site.
Since Florence’s inception in 2014, we’ve solved site-specific challenges first. Our site-forward approach has allowed us to thoughtfully expand our customer network and suite of products to address all stakeholders – research sites, sponsors, and CROs.

Together we are a globally connected and collaborative research network centered on our leading Electronic Investigator Site File, Florence eBinders. Our eBinders solution is in use across 44 countries by more than 10,000 research sites and accessed by prominent sponsors and CROs through Florence SiteLink™ and Florence eTMF.
It is from this perspective that Florence, like any technology vendor, is responsible for driving global compliance and security standards for its customers.
The Rise in Global Clinical Trials
There are many drivers for an eClinical provider to refine its global outlook, infrastructure, and processes. However, the primary catalyst is the steep increase in global trials and research. As shown on ClinicalTrials.gov, for all studies registered, “the number of registered clinical studies in non-U.S. areas stood at 172,209 or 50% of all studies worldwide.” 1
Another factor underscoring a rise in global trials is an increasing shift in business models to strategically address global access to health.
An analysis and report by The Access to Medicine Foundation analyzed 20 leading pharmaceutical organizations’ global research strategies over ten years and found that “the number of companies setting goals and targets related to access, and now also implementing clear, long-term strategies for improving access, has reached 17 [out of 20].” 2
Compliance Needs for Global Technology
As the clinical research profile has evolved, so too must its technology. COVID-19 has further illuminated a focus on global health, as well as demanded clinical research technology vendors to refine processes, infrastructure and know-how. For example, as Florence is committed to our customers’ compliance and security around data and documents, we ensure that we continuously view these standards through a global lens.
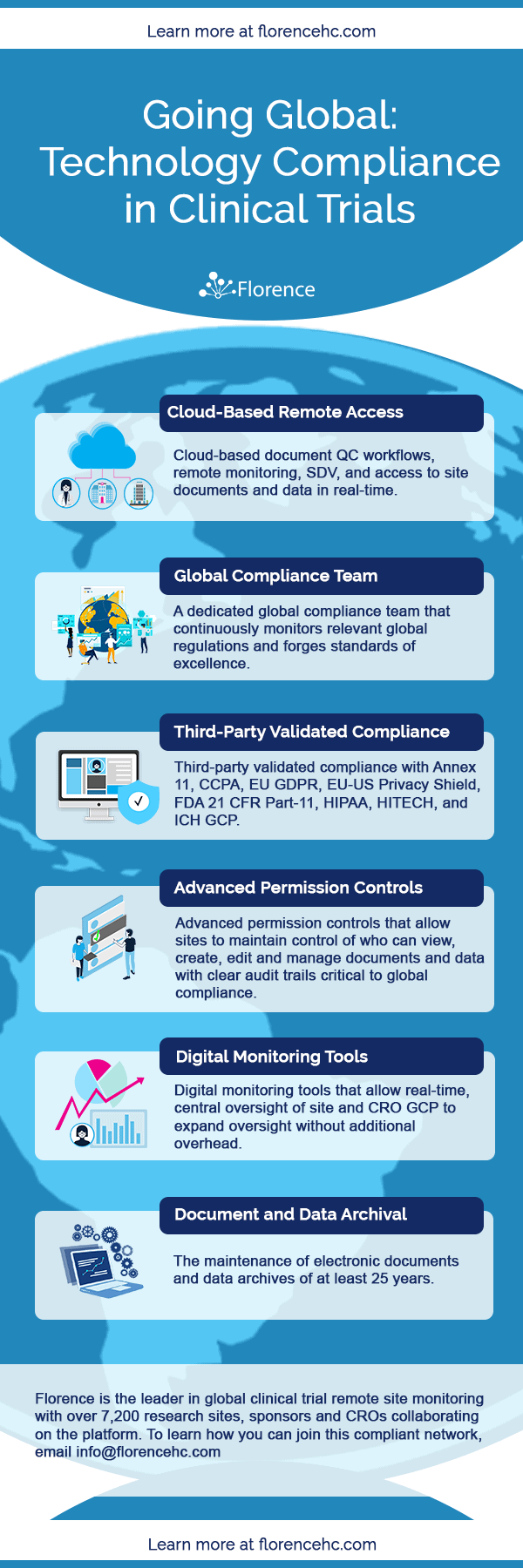
Here are just a few examples of how your vendors should elevate globalization standards and processes to help drive compliance as you conduct global research. They should offer:
1. Transparency as to its technical infrastructure and globalization efforts, security measures and protocols.
2. Cloud-based document QC workflows, remote monitoring/remote SDV, and remote access to research site’s documents and data in real-time or managed across multiple time zones with collaboration queries and clear audit trails.
3. Global instances of data storage centers to ensure compliance.
4. A dedicated global compliance team continuously monitoring relevant global regulations and forging standards of excellence.
5. Third-party validated compliance with Annex 11, CCPA, EU GDPR, EU-US Privacy Shield, FDA 21 CFR Part-11, HIPAA, HITECH, and ICH GCP.
6. Advanced permission controls that allow sites to maintain control of who can view, create, edit and manage documents and data with clear audit trails critical to global compliance.
7. The ability for sponsors to oversee, “…any trial-related duties and functions carried out on its behalf, including trial-related duties and functions that are subcontracted to another party by the sponsor’s contracted CRO(s),” per ICH GCP E6(R2).
8. Digital monitoring tools that allow real-time, central oversight of site and CRO GCP to expand oversight without additional overhead.
9. The maintenance of electronic documents and data archives of at least 25 years.
As the global clinical trial’s evolution continues to take shape more rapidly than ever, it is vital to focus on how changes affect the research site and study stakeholders. As a solution provider that empowers research sites through technology solutions that allow for peak performance, we would highlight the gravity of ensuring this work is protected and that all stakeholders can collaborate effectively – across organizations, roles, and times zones.
SOURCES
1 ClinicalTrials.Gov, Percentage of Registered Studies by Location (as of July 22, 2020), https://clinicaltrials.gov/ct2/resources/trends#LocationsOfRegisteredStudies
2 The Access to Medicine Foundation, First Independent Ten-Year Analysis, “Are pharmaceutical companies making progress when it comes to global health?”, May 2019, https://accesstomedicinefoundation.org/media/uploads/downloads/5d93329e141cb_Access-to-Medicine-Index-10-Year-Analysis.pdf