Florence Trial Operations Platform
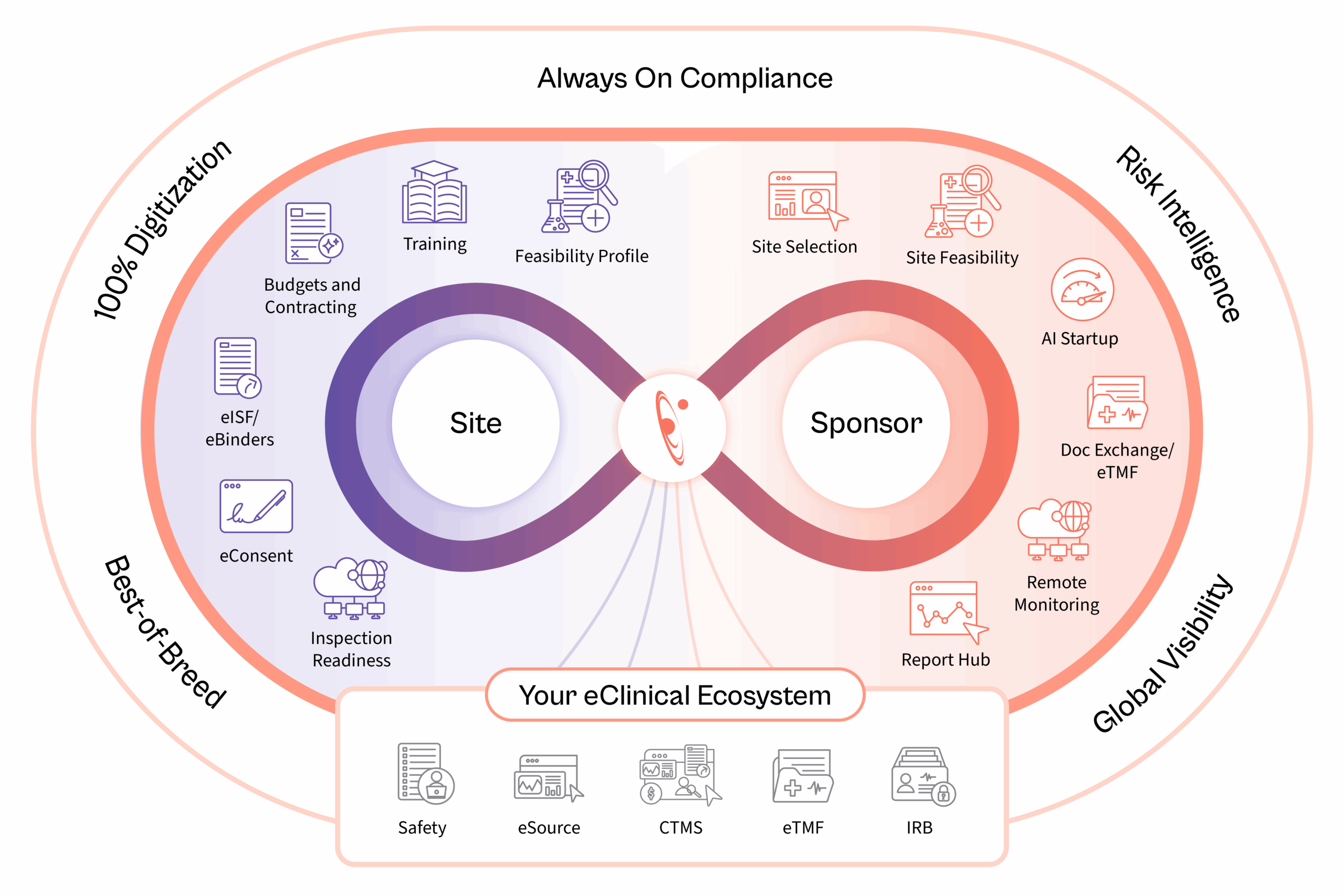
How Florence Transforms Operations For:
Global Visibility.
65k
Trial Sites
600+
Sponsors Supported
90+
Countries
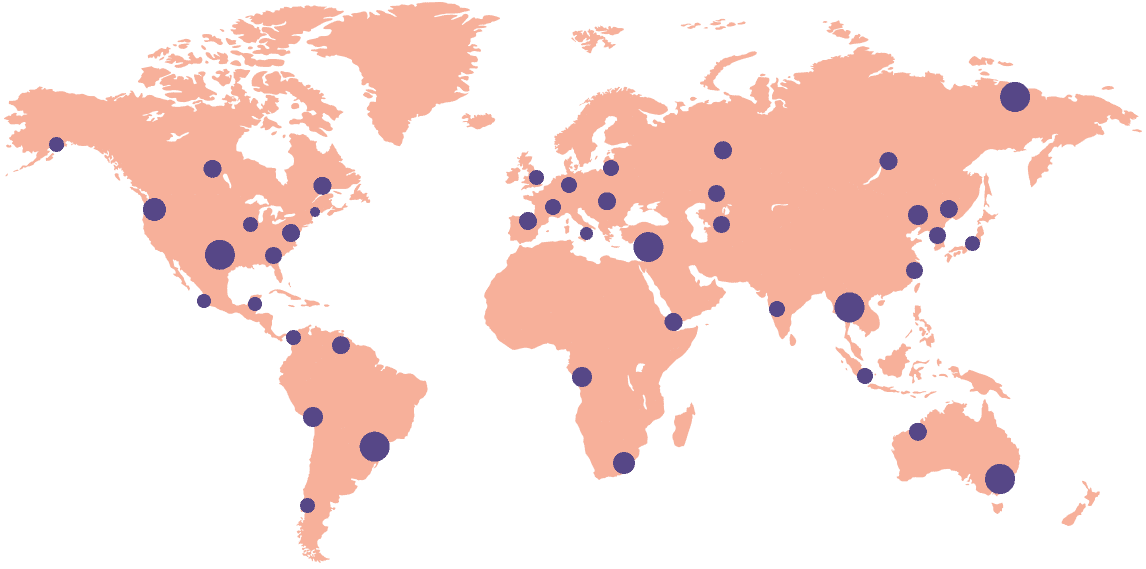
Florence Trial Operations Platform

How Florence Transforms Operations For:
Global Visibility.
65k
Trial Sites
600+
Sponsors Supported
90+
Countries
