This year we’ve met with 45 pharmaceutical, biotech, and device sponsors about both risk-based and remote-based monitoring (the combination referred to as RBM), and we’re seeing sponsors migrate to RBM in very different ways. We encountered three main RBM positions:
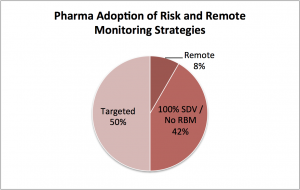
- Targeted. 50% have moved to targeted monitoring based on EDC data analysis. This means their monitoring efforts using traditional techniques are reduced or redistributed to targeted sites, documents, or patients. A key element of this approach is reducing source data verification (SDV), the process by which clinical source documents are reviewed and compared with what’s in the central EDC database.
- 100% SDV. Most of the remaining 42% are fearful of the inherent risk in reducing SDV as part of targeted monitoring. They have chosen to stick with historic monitoring approaches for now.
- Remote. Meanwhile, a small but increasing number of sponsors (~8%) are developing a targeted plus remote SDV approach. This delivers the best of both worlds – cost savings through reduced site visits and the security of 100% SDV.
Why this distribution? Let’s look at how the industry got started with RBM. First, the FDA & EMA’s guidance in 2013 provided much needed monitoring cost and time relief for sponsors. We’ve seen a meaningful (~50%) percentage of the industry begin the process of testing RBM in their trials within the past two years. About half of those transitioned to RBM on the majority of their phase 2+ studies. Sponsors we’ve met with say the benefit in reduction of both cost and time to closeout is real.
Learn more about how sponsors are reducing both cost and time from study start to closeout.
With those benefits, why has only half of the industry moved to RBM as a core strategy? We believe the answer comes down to remote data access. Most sponsors embracing RBM rely on just one data tool in support of RBM — the EDC database for risk analysis. Trouble is, this only provides analysis of data points entered by the sites. This targeted approach ignores the mountain of data available via regular on-site SDV access. Simply put, a meaningful portion of sponsors (in group 2 above) are uneasy with this tradeoff.
So, 42% of those we met with still insist on 100%, in-person SDV for their studies. How can they move beyond this? Remote SDV as part of a broader RBM strategy is the solution. A remote monitoring approach can help this side of the market realize the benefits of RBM. About 8% of the sponsors we met with have started down this path.
These 8% won’t be in the minority for long. In fact the FDA, EMA, and TransCelerate guidances all recommend a hybrid of risk and remote activities in line with this approach. It’s this blend that will make RBM real for the other 50% of the industry, and we’re building tools to help. Learn more about how sponsors are using eBinder Suite to tackle these new cost-saving approaches to the industry.