How to Use Patient Engagement Technology in Your Clinical Trials
Clinical trial sites embraced remote patient engagement technology in 2020 and 2021. 95% of research sites now offer telemedicine visits, while 94% use electronic patient diaries and 91% use electronic informed consent documents. All of these forms of tech keep patients involved in their own healthcare, which can increase clinical trial recruitment and retention rates.
Patient engagement technology plays a critical role in patient-centric clinical trials, trials that try to increase recruitment and retention by improving participants’ experiences. Resources like online diaries and telemedicine calls can help participants understand their treatments and join trials without going to the site for every visit.
But not all participants are comfortable with tech. Your site needs to guide participants through how to use software to see the increases in participant recruitment and retention that you’re hoping for. We’ve compiled a list of strategies to help you make the most of any patient engagement technology you use for your trials.
Benefits of patient engagement technology for clinical trials
If using patient engagement technology requires additional training and planning, why bother? Although many sites adopted participant technology because of COVID-19, remote tech isn’t only useful during a pandemic.
86% of research sites in our Mid-Year 2021 Survey said they were using at least one form of remote technology or planned to use remote tech in 2022. Given this tech’s role in improving patient outcomes, recruitment, and retention, it’s no surprise more and more sites are embracing it.
1. Help patients take charge of their health
When patients have easy access to information about their health, it lowers their rates of hospital admission and improves their health outcomes. In one study, patients who received personalized health guidance from nurses had 30% fewer emergency visits and readmissions to the hospital than patients who didn’t.
83% of patients say they would like regular reminders from their physicians or nurses about activities like checking their blood pressure, taking medication, doing physical therapy exercises, or scheduling follow-up appointments. Research sites can better serve their participants by providing this service. With permission, you can send participants automated texts or email reminders about trial activities. By thinking of participants as customers who deserve exceptional service, you can encourage them to complete the trial, participate in future trials, and even talk to their friends about signing up for trials.
Patient engagement technology can also keep participants informed in a language they’re comfortable with. For example, one hospital in El Paso, TX used software that automatically translated at-home care instructions into multiple languages. This allowed nurses who didn’t speak a patient’s first language to give that patient accurate health information.
Having instructions in their first language lowered patients’ hospital readmission rates from 16% to 7%, and offering information in multiple languages can make participants feel more included.
Clinical trials can imitate this study’s success and use patient engagement technology to communicate with participants in their own languages.
2. Increase participant recruitment
Buying new software can be expensive. But patient engagement technology can save sponsors money in the long term by increasing recruitment rates.
85% of clinical trials fail to recruit enough patients, and 80% are delayed due to recruitment problems. Patient-centric trials dramatically cut down on recruitment delays. Trials that offered patient-centric features took 4 months to recruit 100 participants, while non-patient-centric trials took 7 months to recruit the same number.
Patient engagement technology can also help sites recruit more diverse patients. In one survey, 58% of Hispanic patients and 53% of African American patients said they’d like to receive clinical trial information on their smartphones, versus only 39% of white patients. This is notable because Latinx and Black patients are often underrepresented in clinical trials.
Patient-centric trials that use remote technology also work better for participants who have hourly jobs, who live far away from the research site, or who have disabilities. Participants with strict work schedules can’t always take time off for visits to the clinical trial site, especially if they have to drive for hours to get there.
Participants who can’t drive because of age or disabilities may also have trouble visiting the research site frequently. If they can sometimes use patient technology to check in with the site from home, clinical trials will become far more accessible for them.
The patient engagement technology you can choose from for your trials
Patient engagement technology can make it easier to recruit participants and help them stay informed. But first, research sites need to choose the right software for their trials and their participants.
Remote patient engagement technology comes in many forms, from simple telehealth calls to wearable sensors that transmit participants’ data on a constant basis. However, some forms of this tech are experimental and won’t work for all trials or all patients.
If your site embraces multiple forms of advanced technology at once, trials could become more difficult for patients instead of more patient-centric. Not every trial participant has WiFi, a smartphone, or a computer, and many participants aren’t comfortable using tech, as clinical trial diversity expert Leslie Byatt pointed out on our podcast.
You can alleviate these problems by using a “crawl, walk, run” approach. Rather than embracing multiple forms of complicated technology, start with a few pieces of technology that offer a high return on investment (ROI), that are easy for your staff to explain, and that participants will enjoy using. You can also offer non-technology options for participants who don’t have Internet access or devices.
If you decide to try out a few pieces of patient engagement technology, you’ll have an abundance of options to choose from. Here are a few types of software you might want to consider:
1. Recruitment software
Recruitment software lets participants take the initiative and search for clinical trials that meet their needs. These programs can help sites shorten recruitment timelines.
2. Retention software
Retention software helps patients keep participating in the trial once they’re enrolled. This category could include electronic diaries and software that reminds patients to take their medication, do their physical therapy exercises, or wear their devices.
3. Data management software
Data management software collects the hard numbers from clinical trials: lab results, clinical assessments, and digital biomarkers. Wearables, electronic data capture (eDC), electronic clinical outcome assessment (eCOA), and electronic patient-reported outcome (ePRO) platforms all belong in this category.
If you choose software that requires patients to use wearables or log in and record their own data, you’ll need to provide more training to ensure they understand how to use the technology correctly.
4. Informed consent software
Informed consent is one of the fastest growest areas of patient engagement technology. 57% of research sites already use electronic consent (eConsent), and 18% plan to use it in the future. eConsent technology lets participants review consent forms on their own computer or device so they can enlarge the text, reread multiple times, or share the document with their family before they sign.
How to introduce participants to patient engagement technology
Once you choose which patient engagement technology you want to try, you need to develop a change management plan. One training session may help participants and site staff use simpler technology, like an eConsent platform. But for more complex technology, like ePRO and wearables, you’ll need a more in-depth strategy.
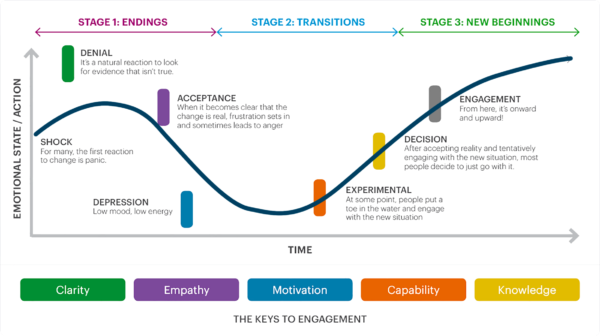
At Florence, we use a modified Kubler-Ross change management strategy. Our strategy focuses explicitly on adjusting to technology and consists of six steps: denial, acceptance, depression, experimentation, decision, and engagement.
We’ve successfully used this strategy to introduce thousands of research sites, sponsors, and CROs of all sizes to new technology. To learn about this process in more detail, check out our guide to change management.
1. Denial
The participant finds the new technology intimidating. They may wonder why they can’t just use paper or question whether they want to be in the trial. Not all participants go through this stage, but older participants or participants who rarely use technology might.
How you can help: You can fight denial with clarity. Explain why you’re using the technology and how it will make life easier for the participant, not just for your site. Some participants may also like to hear about how the data gathered using tech could advance medical research.
2. (Grudging) Acceptance
When we talk about acceptance, we don’t mean that the participant is fully on board. We mean that they’ve agreed to use the technology, but they might still be nervous or upset about it.
How you can help: Lean into acceptance with empathy. Listen to the participants’ concerns and address them if you can. This is also a good time to show the participant the training plan. Will they just need one training session? Several? Do you have videos or step-by-step guides to introduce them to the tech?
3. Depression
Once the trial starts, some participants will eagerly begin using patient engagement technology. But others may get stuck in the depression phase, using the software reluctantly and infrequently. They could miss telehealth check-ins and data submissions or even become lost-to-follow-up.
How you can help: Motivation and support from others can defeat depression. Try getting the participant’s care team or the staff at the research site on board with the software. A “tech champion” who’s genuinely excited about the software can help the participant understand it and get excited too.
4. Experimentation
At this stage, the participant is beginning to explore what the technology can do. If they’re using a recruitment app, they may start searching for other clinical trials to join in the future. If they’re using an electronic diary or patient log, they’ll explore all of the features it has and update it regularly.
How you can help: Offer ongoing training and support. Make sure each participant knows how to use the technology comfortably. Answer questions or provide personalized training sessions if they don’t.
5. Decision
The participant feels comfortable using the technology and has decided to keep doing so. They’re fully invested in completing both the in-person and technological parts of the trial.
How you can help: Keep patients involved by sharing whatever feedback you can about how the study is going and the eventual impact their results will have. Let participants share feedback about the study procedures and what would make studies work better for them.
6. Engagement
The participant isn’t just using technology–they’re enthusiastic about it. They understand how eConsent, patient diaries, telehealth calls, and other forms of remote technology can help them and other participants. They freely offer feedback on how to make future trials better and are eager to join a trial again.
How you can help: Encourage patients to share their trial experiences with loved ones or acquaintances. Let the participant know if they’re eligible for future trials and what forms of patient-centric technology you’ll be using in those trials.
Planning for the future with patient engagement technology
When used strategically, patient engagement technology can make it easier for participants to join clinical trials. Research sites could see their recruitment rates rise and find better-informed and more diverse patients.
But if you choose technology that’s too complex or try to introduce too many new programs at once, participants might be pushed away from trials instead of drawn to them. Browse our guide to decentralized trials for more information about how to make remote technology work for your participants and your research site.
References
Considerations for improving patient recruitment into clinical trials. Retrieved September 9, 2021, from https://www.clinicalleader.com/doc/considerations-for-improving-patient-0001.
Dietsche, E. (2019, February 19). Survey: When communicating with a physician, patients prefer secure texting to patient portals. MedCity News. https://medcitynews.com/2019/02/survey-physician-patients/.
Keens, M. (2019, September 3). Why patient-centric clinical trials are easier said than done. Applied Clinical Trials. https://www.appliedclinicaltrialsonline.com/view/why-patient-centric-clinical-trials-are-easier-said-done.
Krames Patient Education (2010). Reducing Hospital Readmissions with Advanced Patient Education. Yardley, Pennsylvania; FierceHealthcare.
Paraxel. (2019, January 29). Decentralized clinical trials: Are we ready to make the leap? BioPharma Dive. https://www.biopharmadive.com/spons/decentralized-clinical-trials-are-we-ready-to-make-the-leap/546591/.
Research America (2017). Clinical Trials: Survey Data of Minority Populations. Arlington, Virginia; Research America.
Sharma N. S. (2015). Patient centric approach for clinical trials: Current trend and new opportunities. Perspectives in clinical research, 6(3), 134–138. https://doi.org/10.4103/2229-3485.159936

