How to Create a Digitally Connected Clinical Trial Ecosystem
Sponsors and CROs’ ability to access and collaborate with a research site is essential to the success of every study. Each study phase and clinical operations process requires the ability to access, review, and exchange documents efficiently and promptly.
The speed and accuracy at which this exchange occurs can critically affect study timelines and overall trial success.
A disconnected mix of on-site visits, email communications, and portals restrict site access and document exchange for Sponsors and CROs alike.
Sites are making significant investments in purpose-built Electronic Investigator Site File (eISF) platforms to streamline their internal operations and mitigate costly delays, with 63% of all sites planning to have an eISF by the end of 2020, according to our 2020 State of the Industry Report.
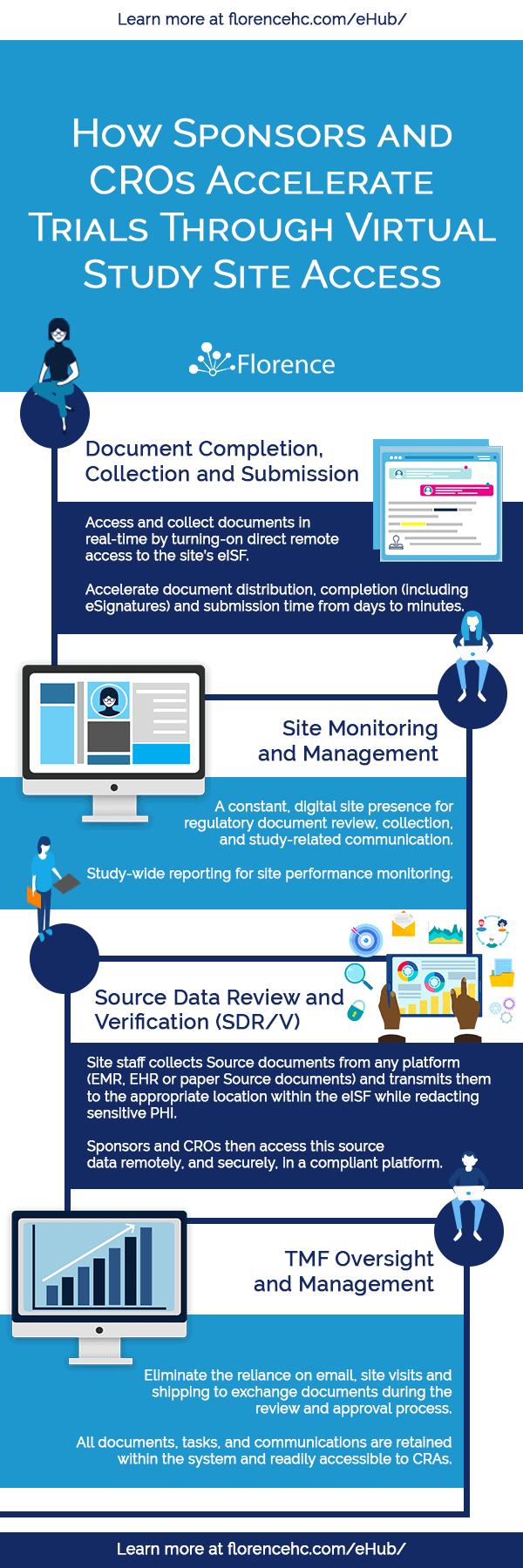
As study sites “come on-line,” Sponsors and CROs are in a unique position to harness this digitally connected network of sites to turn on remote site access and Source document review/verification (SDR/V), and streamline workflows.
Florence is on the leading edge of innovation and transformation, currently powering thousands of sites with its eBinders platform for eRegulatory/eISF and is connecting Sponsors and CROs to their sites via Florence SiteLink™ for remote monitoring.
Florence SiteLink is a collaborative workspace, providing unprecedented levels of remote site access and document quality control that ultimately decreases study cycle times while increasing data quality.
In this article, we examine the key quality outcomes and technology recommendations associated with digitally accessing the site eISF.
You can also access the white-paper Harnessing the eISF here, which outlines each process including common challenges and transformative solutions.
Integrating Site Connectivity to the Clinical Trial Process Map
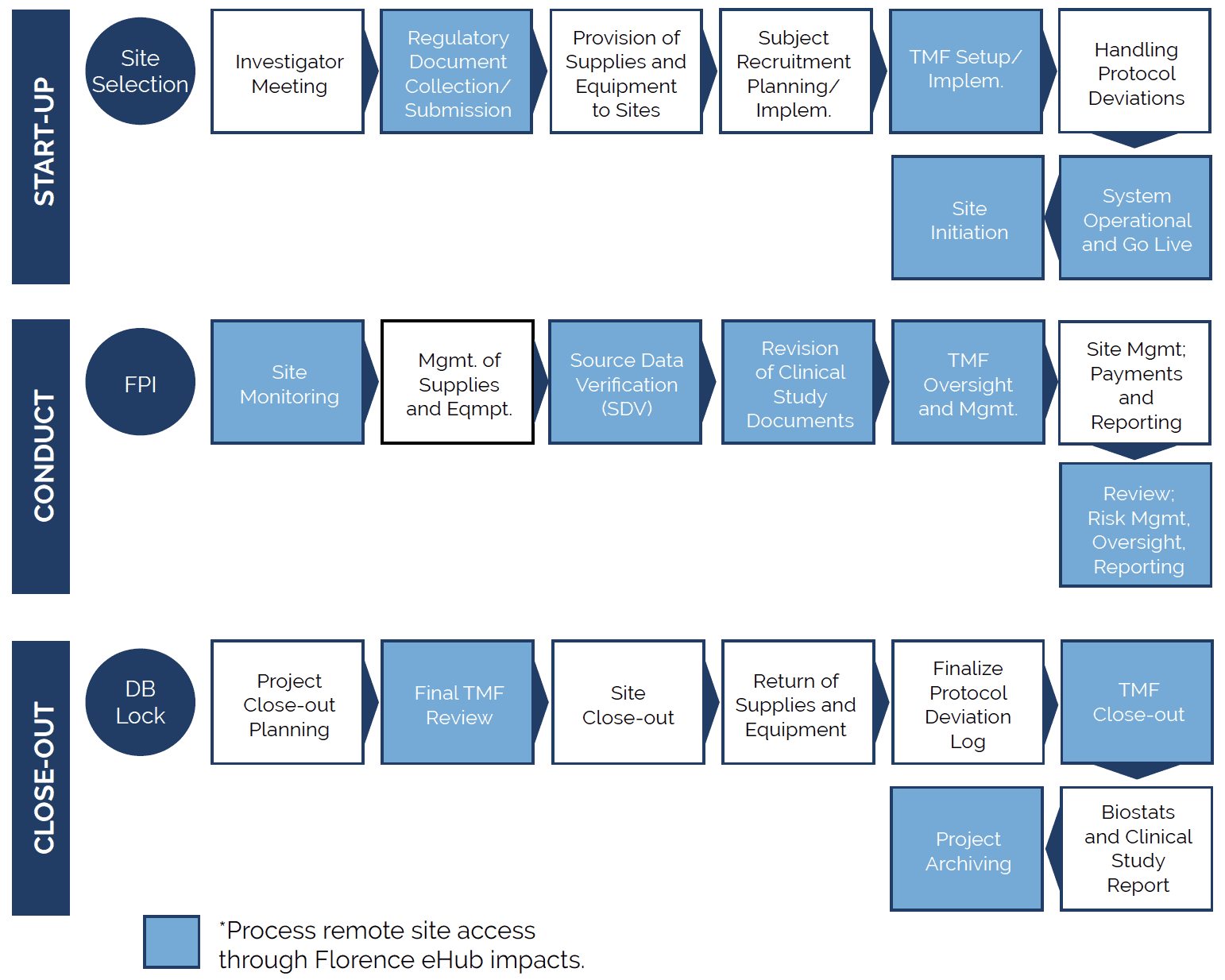
Creating seamless integration across sponsor, CRO, and site digital platforms starts with examining the Clinical Trial processes that utilize each platform and identifying challenges within the current system.
Clinical studies typically follow three distinct phases: Start-up, Conduct, and Closeout. Within each of these phases are essential processes to ensure the study is on track and progressing.
Integrating the site’s eISF platform with the Sponsor/CRO workflows through digital connectivity, like Florence SiteLink™, delivers a direct impact on numerous core processes requiring site document and data access, review, and exchange.
To help you better identify the most impacted clinical study process challenges, we highlighted them in the overview map below. We also cover these processes in more detail in the following article.
Key Takeaways and Recommendations
Sponsors and CROs can rapidly accelerate clinical trial timelines and reduce the overall cost by digitally connecting to platforms that Sites already know and love.
The key takeaways and recommendations for creating a digitally connected ecosystem are listed below.