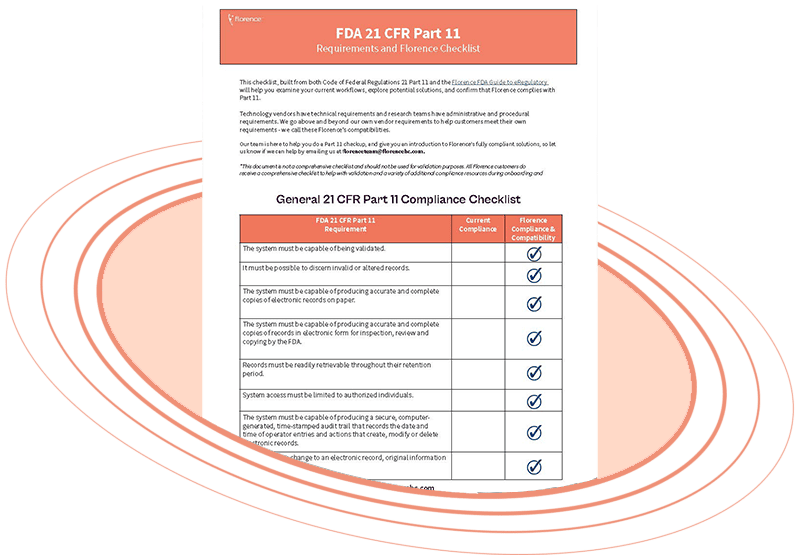
FDA 21 CFR Part-11 Compliance Checklist
Looking to evaluate technology solutions for your clinical trial but unsure if they meet FDA 21 CFR Part 11 requirements? Our Checklist for Evaluating Technology in Clinical Trials has got you covered!
Our checklist includes:
- Essential assets you should look for in technology solutions to meet FDA 21 CFR Part 11 requirements
- Expert advice to help you make an informed decision
- A comprehensive list of questions to ask technology solution providers
We understand that every platform is unique, which is why our checklist is tailored to ensure you get the best possible technology solution for your clinical trial needs.
Ensure compliance with FDA 21 CFR Part 11 requirements in your clinical trial with our Checklist for Evaluating Technology in Clinical Trials today