The rate of cancer death has been decreasing, and people are living longer with cancer than ever before. Approximately 64% of US patients diagnosed with cancer in 2005 have lived 10 years or more beyond diagnosis, up from 35% for those diagnosed in 1975. — American Society of Clinical Oncology Cancer Advances Report, 2018.
This is real progress and overall ASCO’s report is encouraging. A key part of progress are the 18 targeted, immunotherapy drugs and 13 new treatment modalities that cleared FDA approval in 2017—bettering outcomes for patients with 16 different types of cancer.
But we can do better.
On average, these studies took 6 years to complete. For the 1.7 million people receiving a cancer diagnosis each year, this is an eternity.
So how do we get new oncology drugs to market faster? One way is by eliminating non-value added tasks during their clinical trials since trials are the last gate before new drugs can hit the market.
Let’s look then at one category of clinical trial delays—study startup. Startup encompasses all the tasks required (per the FDA) of a clinical trial site before the first patient can be enrolled. A study by the University of South Florida showed that the average industry-sponsored study took 76 calendar days to get started. Extrapolating this across the 31 drugs approved in the last year, 6.5 years were wasted in oncology study startup.
Let’s dig in more. The study startup process comprises 5 sub-processes, 30 activities, 11 decision points, 5 loops, and 8 participants. It’s reasonably complex.
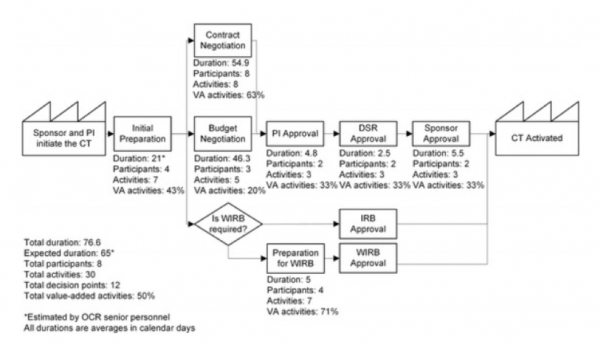
Process Diagram from Activating clinical trials: a process improvement approach.
The USF research team discovered that, of all those components, budget negotiation and contract negotiation are the most wasteful process, each taking 46 and 55 days, respectively, but more importantly harbor only 20% and 63% value-additive admin activities. Said differently, 58% of research teams’ effort is wasted during the key parts of study startup.
How can we fix this?
The USF team recommends three strategies:
- Parallelization of activities within the administrative process and
- Increased administrative capacity for critical processes.
- The use of Master Agreements to reduce the time spent on Contract Negotiation and Budget Negotiation
At Florence we’re supportive of all three approaches. In particular, Florence eBinders help with recommendations #1 (by helping teams project manage and parallelize startup processes) and #2 (by automating wasteful startup tasks with software).
How? In Florence’s internal tests, clinical researchers were able to complete key startup tasks 40% faster using eBinders over paper and spreadsheets. The impact of eBinders materially compresses wasted effort in startup, not only delivering startup faster but allowing research teams to take on more studies with the same staff. New therapies get to market sooner as a result.
So, as ASCO reports, we’re making progress but we’re not there yet. With 1.7 million patients waiting for breakthrough cancer therapies each year, Florence’s goal is to give research teams productivity, and in doing so get the new drugs to market faster.
Click here to learn more about eBinders and explore how they can help you make progress.

