Sites, sponsors, and CROs now perform the bulk of their clinical trial operations by logging into cloud-based systems.
The rapid shift to digital document and data management solutions opened the door to innovative virtual collaboration solutions like Florence SiteLink™. Florence SiteLink provides sponsors and CROs direct digital connectivity and collaboration access with their sites via connecting to the largest Electronic Investigator Site Files (eISF) in research.
In this article, we outline the impact on the below study processes:
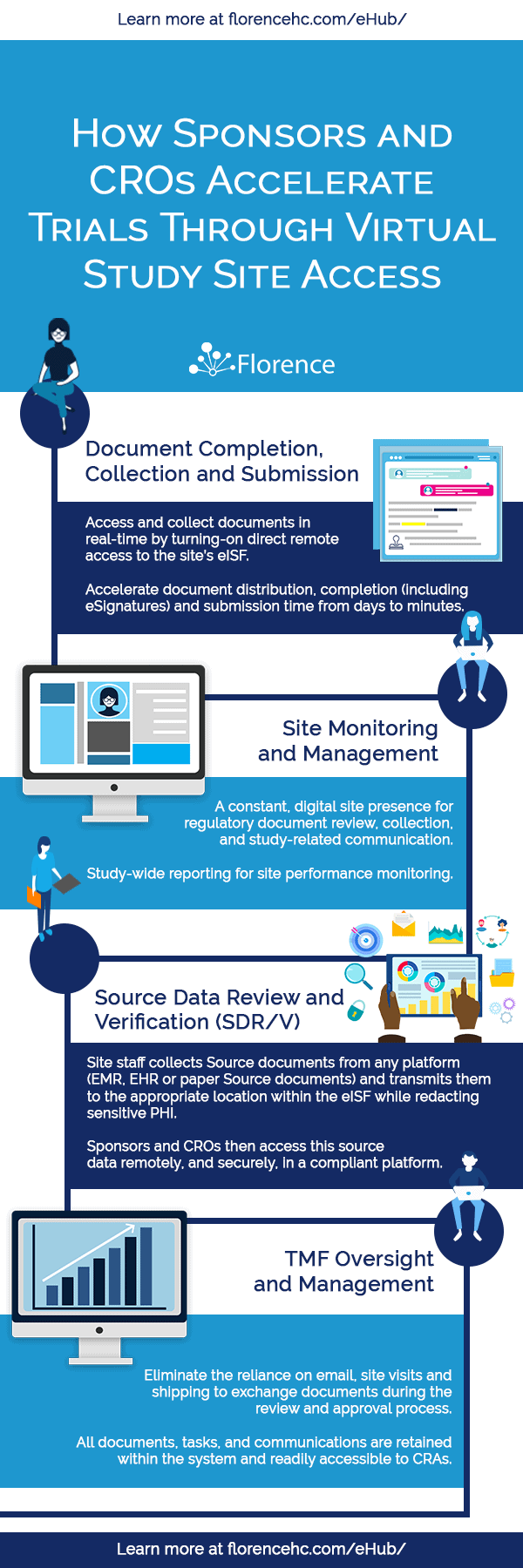
- Document Completion, Collection and Submission
- System Set-up, Go-Live, and Site Initiation
- Site Monitoring and Management
- Source Data Review/Verification (SDR/V)
- TMF Oversight and Management
- Project Archiving and TMF Close-out
You can also access the complete whitepaper, “Harnessing the eISF”, which outlines each process and additionally includes key recommendations and quality outcomes of digitally accessing the site eISF.
How to Eliminate Clinical Trial Bottlenecks through Site Access
Clinical Trial Document Completion, Collection and Submission
Regulatory document collection and submissions from sites are a bottleneck for sponsors and CROs, often prolonging study start-up timelines and increasing the overall cost.
Contribution to this bottleneck include:
- Reliance on in-person visits, email, or one-time use portals resulting in essential document delay, misplaced files, and imperfect quality copies.
- Inconsistent binder distribution and document collection processes across sites.
- Investigators and site staff that are slow to complete and submit documents because of belabored internal workflows.
- Sponsors and CROs must rely on relationships and anecdotal information, not actual site performance data, to select sites.
eClinical System Set-up, Go-Live, and Site Initiation
The introduction of new technology at a site by Sponsors and CROs often results in low adoption and minimal return on investment caused by:
- Disruption of site workflows caused by the inability to integrate with existing software platforms (CTMS, ePro, EMR, EDC, eTMF, and Portals).
- Investigators and site staff resistance to new technology platforms.
- Added burden on CTA/CRA and IT staff in having to conduct software training and support.
- Compliance, security, ownership, and privacy concerns.
- Poor user interface (UI) design resulting in frustrated users.
Site Monitoring and Management
A critical barrier to accelerating research and increasing the timeliness, quality, and completeness of TMF data is the on-site monitoring visit.
The monitoring of sites is a crucial aspect of the clinical study. It often requires on-site visits, as most sites have not yet employed remote access solutions. These on-site visits lead to significant challenges for the Sponsor/CRO, including:
- Crucial data that is not available until the next monitoring visit, severely limiting the ability to manage sites in real-time, uncover issues, and identify bottlenecks.
- On-site visits that are challenging to schedule and disruptive to the site’s day-to-day operations.
- The difficulty of maintaining visibility into essential study documents, source, and related tasks.
Source Data Review/Verification (SDR/V)
Source Data Review/Verification (SDR/V) is one of the most challenging tasks for Sponsors and CROs to perform remotely.
Each site has its systems, rules, regulations, and processes regarding how Source data is collected, stored, and exchanged.
Reliance on paper files or remotely inaccessible EMR/EHR systems requires on-site visits during which manually scanning, uploading, redacting, and accessing Source data adds to wasted time.
TMF Oversight and Management
Disjointed eISF to eTMF processes result in delays to study completion and submission.
eTMF quality and completeness is a crucial challenge for many sponsors and CROs. These result from:
- Delayed site document uploads due to email systems, mislabeling, and misfiling.
- Poor quality documents due to scanning, shipping, and printing.
- Limited visibility into incomplete site documents and eTMF management status.
Taking Advantage of Site Access Tools
Sixty-three percent of research sites surveyed in the Florence 2020 State of the Industry Report plan to have an eISF platform by the end of 2020.
Sponsors and CROs are well-positioned to take advantage of this convergence of purpose-built technology, a critical mass of over Investigators, and a primary focus on remote connectivity to streamline their clinical operations.
Organizations that take advantage of direct digital site connectivity will be in an advantageous position:
- Accelerating study start-up processes by as much as 40%.
- Enabling remote monitoring and SDR/V while reducing on-site visits.
- Gaining real-time visibility into research site operations.
- Increasing eTMF quality and completeness.
- Potential accelerating regulatory submissions.
Additionally, these sponsors and CROs become more productive and efficient in limited travel and restricted site access.
Florence is a pioneer in this field and the only remote site access, monitoring, and SDR/V platform on the market with a critical mass of over 10,000 Investigators.
Sponsors and CROs must take advantage of this transformation early to ensure business continuity and long-term study success.