Overcoming Four Common Challenges in Multicenter Research
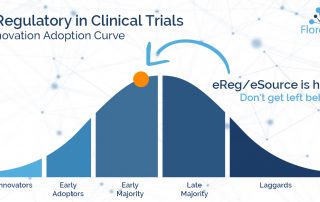
Blake Adams2023-05-03T21:57:47-04:00Overcoming 4 Challenges in Multicenter Clinical Trials Efficiently managing multicenter clinical trials is an essential capability of the modern research center. Complex protocols often require diverse subjects spread across a vast network of research sites - increasing pressure on the coordinating center to streamline operations, maintain oversight, and avoid compliance risks. While the [...]