Solutions > eTMF
Florence’s eTMF
Streamline TMF documents.
Connect TMF to eISF.
Accelerate close-out.
Get set up fast with the top-rated platform in ease-of-use, ease-of-setup, and customer support. Plus, connect directly to your sites with the only eTMF integrated with the industry-standard electronic investigator site file network.
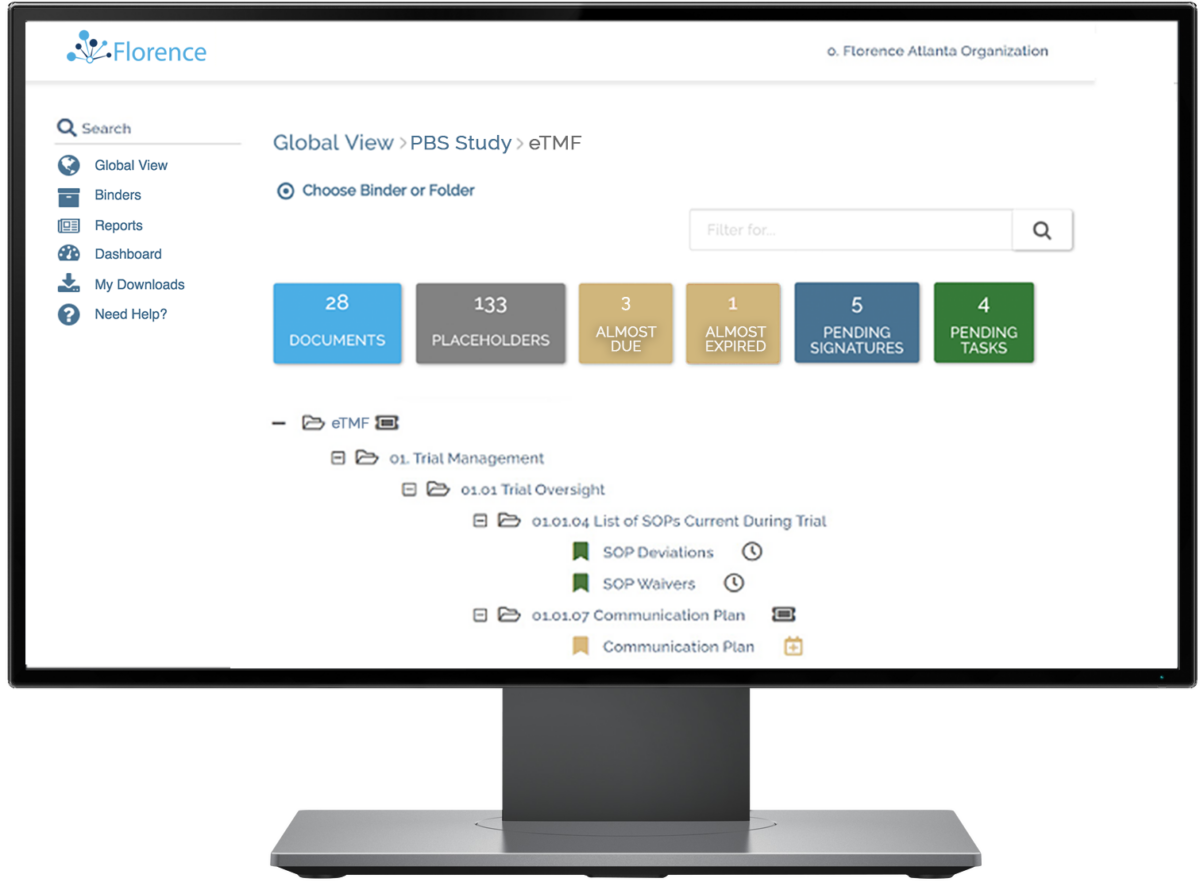
How Florence’s eTMF Transforms Your Operations
Create a digital connection to every site in your study on a platform they already love and use in their workflows.
Go Digital from Start-up to Submission
Create, edit, sign, gather and review trial master file documents from within the platform. Sponsors can also manage all site workflows in one system when connected to the Florence electronic investigator site file.
Simply with Flexible Workflows
Emerging and scaling organizations demand eTMF workflows that adjust to their processes. We don’t force you into a box. You choose how you work in our platform, and you can trust you’re always compliant with global regulations.
Minimize FTE Impact
Complex systems come with complex headcount requirements. Florence is built for ease-of-use and ease of setup, and we’re ranking #1 for them, meaning you need less staff to implement and support your electronic trial master file.
Maximize CRA Efficiency
Connecting Florence eTMF to Florence remote site access allows CRAs to monitor over 60 sites a week with our system. CRA staffing bottlenecks are reduced, travel costs are reduced, and CRAs can make a greater impact on the study sites.
Improve TMF Quality
Integrate SiteLink with your eTMF for seamless document exchange with sites. Boost your eTMF pass rate from 65% to 98.7% like one of our satisfied customers.
Accelerate Start-up Timelines
Automated workflows like electronic logs, placeholders, eSignatures and quality assurance workflows reduce start-up times for most of our customers by 40%.
Accelerate Study Start-up
Intuitive workflows to get your study set-up and activated fast.
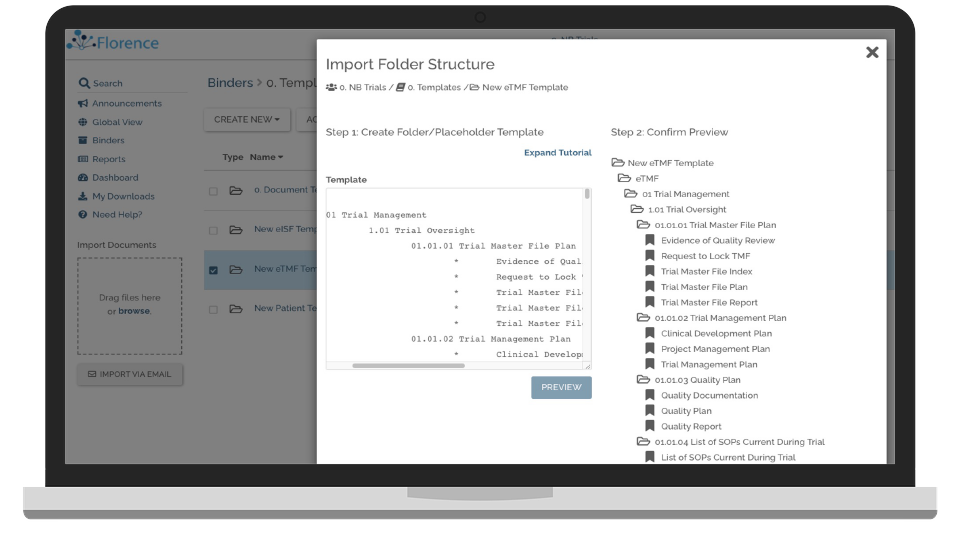
Improve TMF Timeliness and Completeness
Multiple methods of creating and gathering TMF documents combined with advanced dashboards.
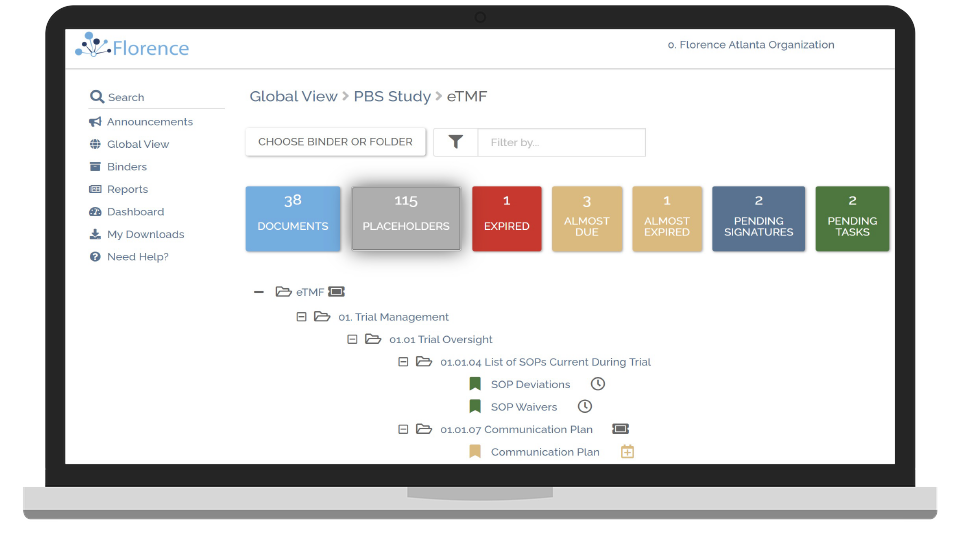
Track Study Performance in Real-Time
Keep track of your entire study and identify risk areas with advanced reports and dashboards.
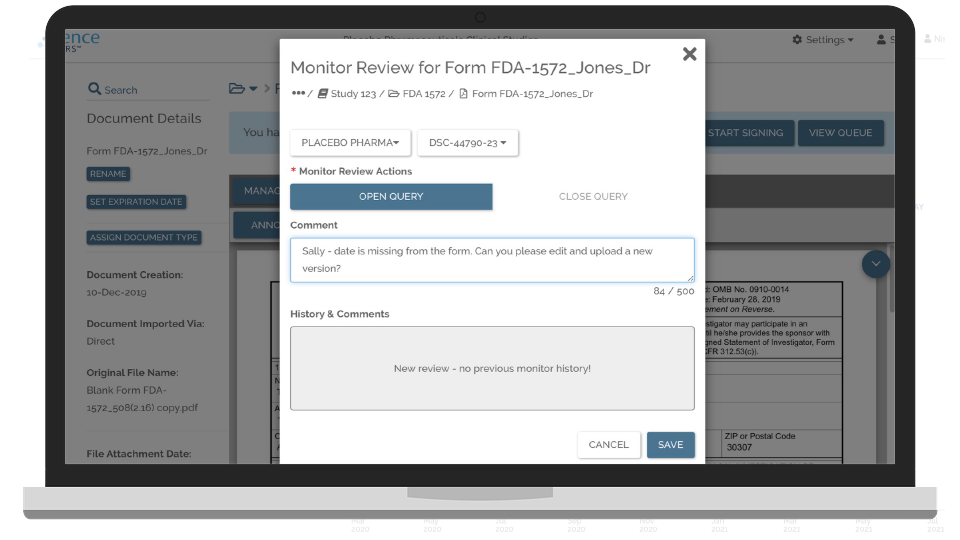
Remotely Collaborate with Study Sites
Deploy and connect to the electronic Investigator Site File (eISF) at every study site.
In clinical research, compliance is crucial. We’ve got you covered globally.