Solutions > SiteLink™
Florence’s SiteLink™
Accelerate timelines. Increase site capacity. Reduce site burden.
Activate remote site start-up, monitoring, and source data review/verification on SiteLink™. Connect and deploy Florence eBinders™, the industry-standard electronic Investigator Site File and Electronic Participant Binder, on a global scale to every site in your study.
How SiteLink Transforms Your Operations
Create a digital connection to every site in your study on a platform they already love and use in their workflows.
Increase Site Satisfaction
Empower your sites with Florence eBinders via SiteLink, the top-rated platform for clinical trial workflows, acclaimed for its user-friendliness, effortless setup, and exceptional support.
Accelerate Study Start-up
Accelerate your drug development process with our automated workflows. Cut start-up times by 40% and reduce study timelines, all while streamlining your workflow.
Decrease Site Workloads
By providing your sites with eBinders, you equip them with a workflow engine that streamlines redundant workflows and consolidates divergent procedures, leading to a reduction of site workloads by as much as 40%.
Expand Patient Access
Embrace the power of digital connectivity and work with any site, anywhere. By deploying remote digital workflows, you can effortlessly expand your network and collaborate with geographically dispersed sites, enabling you to conduct research with greater ease and efficiency.
Maximize CRA Efficiency
Empower your CRAs with remote access capabilities. SiteLink allows for remote monitoring of over 60 sites per week, reducing staffing bottlenecks and travel costs while increasing impact on study sites.
Improve TMF Quality
Integrate SiteLink with your eTMF for seamless document exchange with sites. Boost your eTMF pass rate from 65% to 98.7% like one of our satisfied customers.
“[With Florence’s SiteLink] we’re providing a more valuable site management experience, while allowing more time and opportunities for site support, compliance reviews and continuous monitoring of patient safety and study quality.”
Rajneesh Patil
VP of Clinical Operations and Head of Digital Strategy
IQVIA

Start Up Sites Remotely
Remotely activate and start up your study sites with a complete suite of electronic binder solutions.
“92% of 140 study sites in 8 countries were activated on Florence for remote source access and monitoring in four weeks.”
VP Clin Ops, Top 3 Pharma
“Our highest performing CRAs are now ‘visiting’ 64 sites per week with remote monitoring on SiteLink, up from 2 per week.”
VP Clin Ops, Top 5 CRO
Experience 24/7 Continuous Monitoring of Sites
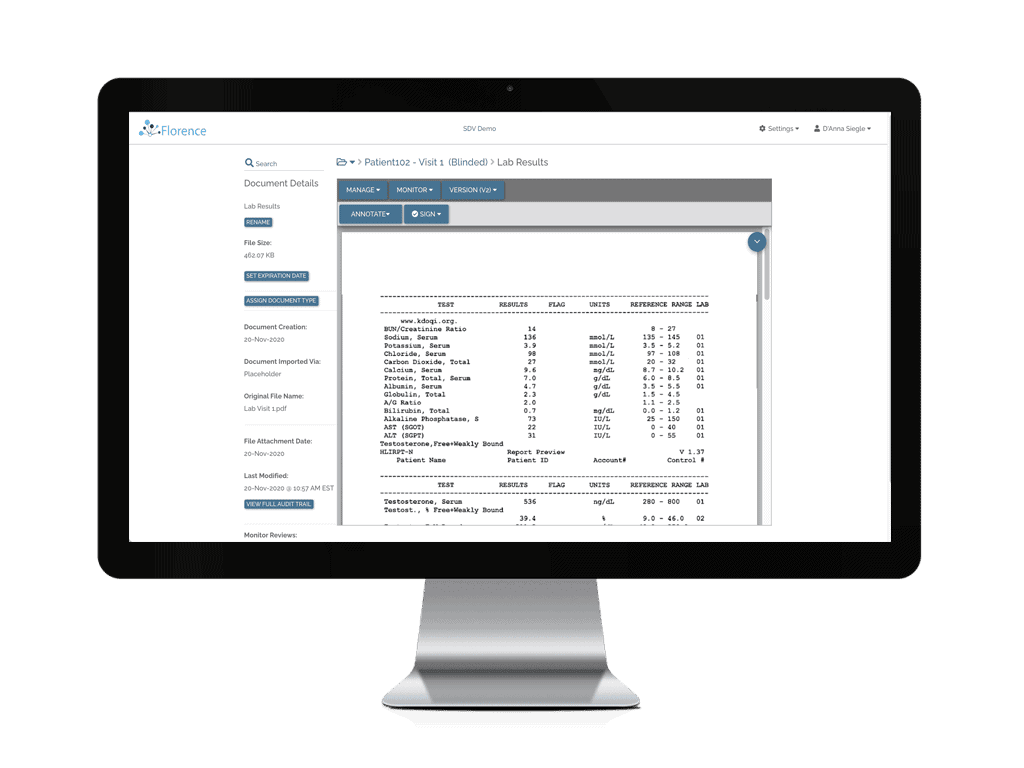
Access your study sites’ Electronic Investigator Site Files anytime for continuous monitoring.
Remote SDV and Remote SDR
Conduct remote source data review and remote source data verification anytime.
“I worked with a site that was supposed to get something done for me in 6 months, a tight timeline. They got it done in 18 days.”
Medical Device Industry Sponsor
“Every day you delay a clinical trial you lose $600K. For a blockbuster drug, that’s 8M per day – that’s just a fact. Florence’s SiteLink saves us 90-100 days on a trial. You do the math.”
VP Site Management, BioPharma
Communicate and Collaborate with Sites Remotely
Enable global dashboards, compliant workflows, communications and electronic logs to remotely manage study site conduct.
Quality Control Documents and Sync Them to Your eTMF
Eliminate the need for mailing, emailing, and faxing study documents or using legacy site upload portals by directly integrating your eTMF with your sites’ eISFs.
“When we start a study there is a regulatory submission … we used to be at a 65% pass rate, now with Florence we are at 99%.”
Director Regulatory, Pharma
In clinical research, compliance is crucial. We’ve got you covered globally.






