It’s Time to Cure the Chaos
Sites are drowning under the demands of today’s clinical research trials, slowing clinical trials, increasing costs, and delaying cures.
62%
Of the research site capacity actually exists to meet patient enrollment targets
78%
More companies have active pipelines than 10 years ago
53%
More clinical trials start every year than a decade ago
41%
Of clinical trials now target complex therapies adding pressure to site operations
Your digital solutions may work for you, but forcing them on sites is a prescription for failure. Duplicate workflows, learning curves, and clunky integrations crush your sites’ progress and keep your discoveries from the patients who need them.
But what if you could enable every site to do their best work?
The Site Enablement Platform
Introducing the Site Enablement Platform for Clinical Trial Sponsors. Our name is almost as long as your trials, and its our job to change that. Florence is designed for what sites need in order to deliver what you need.
SITE START-UPReduce Bottlenecks in Site Start-upActivate sites remotely and equip sites with digital workflows that integrate with their existing workflows. Remotely activate sites and enhance them with digital workflows compatible with their existing systems. Connect with sites efficiently using three methods: Direct integration for sites using Florence, Open API connections for sites with different eISF systems, Deploy our top-rated eISF platform for sites without an existing solution. Easily monitor site performance and activities all in one place. |
SITE CONDUCTSimplify FPI to LPODigitize and integrate every document workflow from first-patient-in to last-patient-out, reducing site burden by up to 40%. Enable sites with the best-in-class eISF (eBinders) and eConsent platform to streamline their operations while activating always-on remote monitoring capabilities, advanced site performance insights, and real-time document exchange with the eTMF. |
SITE CLOSE-OUTSigned, Sealed, SubmittedClose out your studies on time with improved TMF submission quality, compliance, and completeness. Integrate site documents with the eTMF through automated quality control workflows, using seamless methods like API integrations, email, and drag-and-drop. Equip sites with long-term archiving included. |
#1
Rated #1 by sites on G2 for ease of use, ease of setup, and customer support
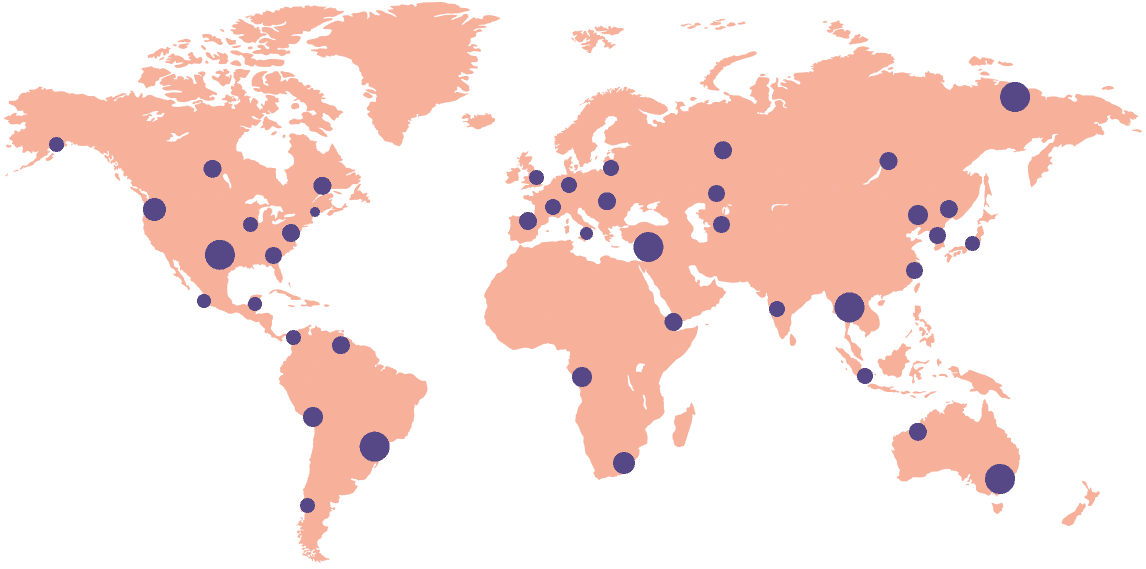
18k+
Sites activated on the platform
55+
Countries connected
92%
Site adoption rate
40%
Reduction in site workload
6.5
Million workflows per month
Make Sites Happy
(CRAs Will Thank You Too)
Enable Sites, Accelerate Trials

E2E Workflow Automation
Manage and automate all workflows in one place. Create, edit, sign, gather and review eISFs, eTMFs, and eBinders all within the platform.

Open Integrations
No need for sites to reinvent their workflows. We integrate with many systems, so sites can work with you while continuing to use their own custom workflows.
Site Intelligence
Gain deep insights into site operations on a global scale with the ability to zoom in on a single document at an individual site. Track compliance and performance with our reporting and analytics dashboards.

Site Activation Teams
Onboarding and adoption take just a few clicks with the help of our Site Activation Teams. Florence is rated #1 on G2 for ease of use, ease of setup, and customer support.

Remote Monitoring
Monitor multiple sites around the world in realtime — all on one dashboard. Now, no protocol deviations, errors, or compliance problems go unnoticed.
Trusted by Sponsors,
Loved by Sites



















